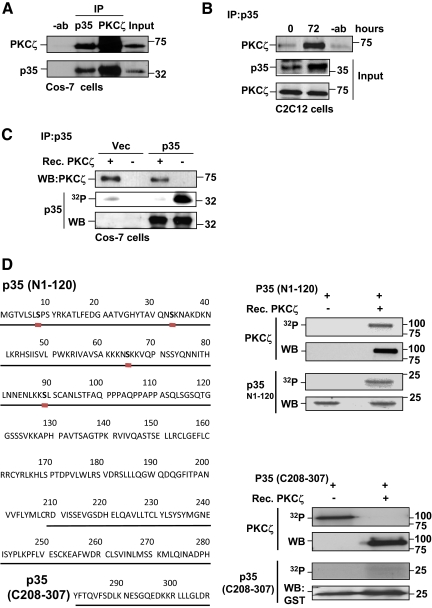
Figure 5.
p35 interacts with and is a target for PKCζ. (A) PKCζ interacted with p35 in Cos-7 cells. Cos-7 cells were transfected with plasmids encoding p35 and PKCζ. Cells were lysed and subjected to immunoprecipitation using either anti-PKCζ or anti-p35 antibodies. Immunoprecipitated samples were separated on SDS-PAGE together with input controls and immunoblotted for PKCζ and p35. The results revealed an interaction between p35 and PKCζ. (B) An interaction between p35 and PKCζ was detected in differentiating myoblasts. C2C12 cells were induced to differentiate, lysed, and subjected to p35 immunoprecipitation. Western blot analysis of immunoprecipitates indicated that p35 and PKCζ interacted during differentiation. (C) p35 was phosphorylated by PKCζ. Cos-7 cells were transfected with p35 or an empty vector. 48 h later, cells were lysed, and p35 was immunoprecipitated. An in vitro phosphorylation assay with recombinant PKCζ was performed using immunoprecipitated p35 as a substrate. The immunoblots demonstrate the amounts of p35 and PKCζ and the autoradiograph the degree of phosphorylation in the different reactions. (D) The N-terminus of p35 contains several putative PKCζ phosphorylation sites (the ProteinScan program was helpful in the prediction of putative phosphorylation sites; see http://156.40.231.198/ProteinScan/ScanProteinForPKCSitesPage.aspx). The N-terminus of p35 was specifically phosphorylated in vitro by PKCζ. In vitro phosphorylation assays were performed with recombinant PKCζ using either truncated N-terminal (N1-120) or C-terminal (C208-307) p35 peptide as a substrate. Proteins were resolved by SDS-PAGE, and the presence of indicated proteins in phosphorylation reactions was monitored by Western blotting. Autoradiographs demonstrate the phosphorylation of p35 peptides as well as the autophosphorylation of PKCζ (indicating kinase activity). Remarkably, only the N-terminus of p35 (N1-120) was specifically phosphorylated by PKCζ.