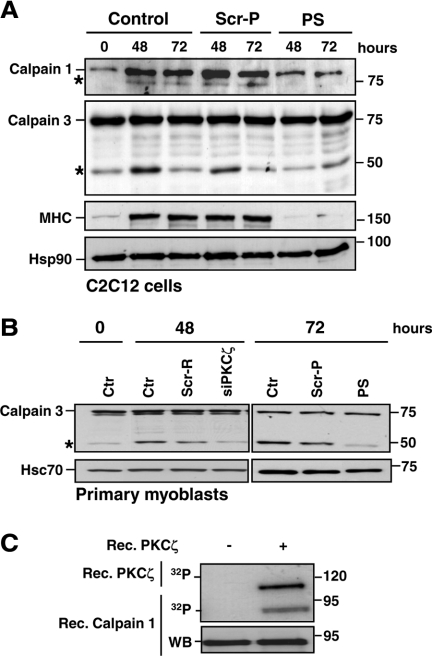
Figure 7.
PKCζ regulates the calpain activity during differentiation. (A) PKCζ depletion inhibited the activation of calpains. C2C12 cells were induced to differentiate in the presence of either the pseudosubstrate inhibitor peptide (PS) or scrambled peptide (Scr-P; 20 μM). Samples were harvested at the indicated time points and analyzed by immunoblotting using the indicated antibodies. The activation of calpains during differentiation was monitored by the formation of the auto-cleavage products (denoted by asterisk) of calpain 1 and the muscle-specific isoform, calpain 3. MHC was used as a marker of differentiation, whereas Hsp90 served as a loading control. The immunoblots indicate significant reduction in calpain activation during differentiation when PKCζ activity was inhibited. (B) Mouse primary myoblasts were induced to differentiate after transfection with PKCζ siRNA (siPKCζ) or scrambled siRNA (Scr-R) or in the presence of the pseudosubstrate inhibitor peptide (PS) or a scrambled peptide (Scr-P). Samples were harvested at the indicated time points and subjected to immunoblotting using the indicated antibodies. Hsc70 served as a loading control. The results verified the observations presented in A establishing PKCζ as a regulator of calpains. (C) Calpain 1 is phosphorylated in vitro by PKCζ. A phosphorylation assay was performed with recombinant PKCζ using recombinant calpain 1 as a substrate. Proteins were separated by SDS-PAGE and transferred to nitrocellulose membranes. First, the phosphorylation of calpain 1 as well as the PKCζ autophosphorylation, indicating kinase activity, were detected by autoradiography and, second, the membranes were subjected to Western blot analysis.