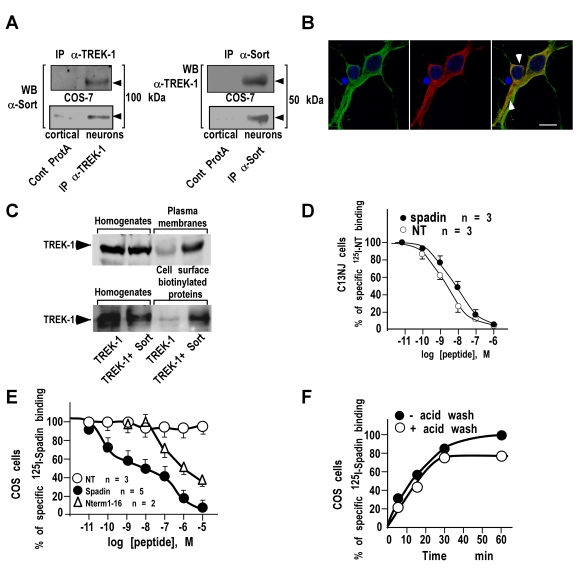
Figure 1. NTSR3/Sortilin and Spadin interact with the TREK-1 channel.
(A) Immunoprecipitation of NTSR3/Sortilin with anti-TREK-1 antibodies (IP α-TREK-1) or of TREK-1 with anti-NTSR3/Sortilin antibodies (IP α-Sort) from transfected COS-7 cells or mouse cortical neurons. Immunoprecipitated proteins were subjected to Western blots and revealed using anti-sortilin (WB: α-Sort) or anti-TREK-1 (WB: α-TREK-1). (B) Double immunofluorescence labeling of TREK-1 (Green) and NTSR3/Sortilin (Red) in mouse cortical neurons. Nuclei were labeled using Dapi (Blue) and co-localized proteins were visualized using merge images (arrows); scale bar, 10 µm. (C) Influence of NTSR3/Sortilin on the expression of TREK-1 at the plasma membranes. COS-7 cells were transfected with TREK-1 in the absence or in the presence of NTSR3/Sortilin. Crude homogenates, purified plasma membrane proteins, or cell surface biotinylated proteins were subjected to Western blot analysis and revealed using anti-TREK-1 antibodies. (D) Competition between 125I-NT and unlabeled Spadin (closed circles) or NT (open circles) for binding to C13NJ cell homogenates. Each point represents the mean of duplicate determinations from 3 independent experiments. (E) Competition between 125I-Spadin and unlabeled Spadin (closed circles), NT (open circles) or N-terminal fragment Gln1-Arg 16 (Nterm1-16, open triangles) for binding to TREK-1 transfected COS-7 cell homogenates. Each point represents the mean of duplicate determinations from 2 to 5 independent experiments. Note that non-transfected COS-7 cells were totally devoid of 125I-Spadin binding. (F) Association kinetics of 125I-Spadin binding to COS-7 cells transfected with TREK-1. At the indicated times, cells were either washed twice with 500 µl of binding buffer (closed circles) or treated with 500 µl of acid-NaCl buffer for 2 min (open circles).