Figure 1.
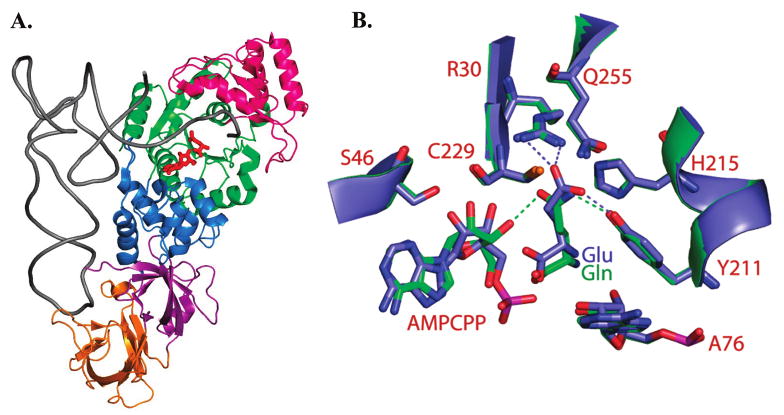
(A) Structure of E. coli GlnRS bound in a quaternary complex with tRNAGln, glutamine, and the ATP analogue AMPCPP (12). The phosphates of AMPCPP are disordered in this structure. The Rossmann fold catalytic domain is colored green, the inserted domain that binds the tRNA acceptor end is bright pink, a helical subdomain following the Rossmann fold in primary sequence is shown in dark blue, and the proximal and distal anticodon binding domains are depicted in magenta and orange, respectively. (B) Superposition of the GlnRS active site in quaternary complexes bound to tRNAGln, AMPCPP and either cognate glutamine (green) or noncognate glutamate (blue). Selected hydrogen bonds are shown as dotted lines. Both bound glutamine and glutamate participate in a hydrogen bond with Tyr211, but only cognate glutamine accepts a hydrogen bond from the 3′-OH group of ATP. Glutamate also makes direct electrostatic hydrogen bonds with Arg30.
