Figure 2.
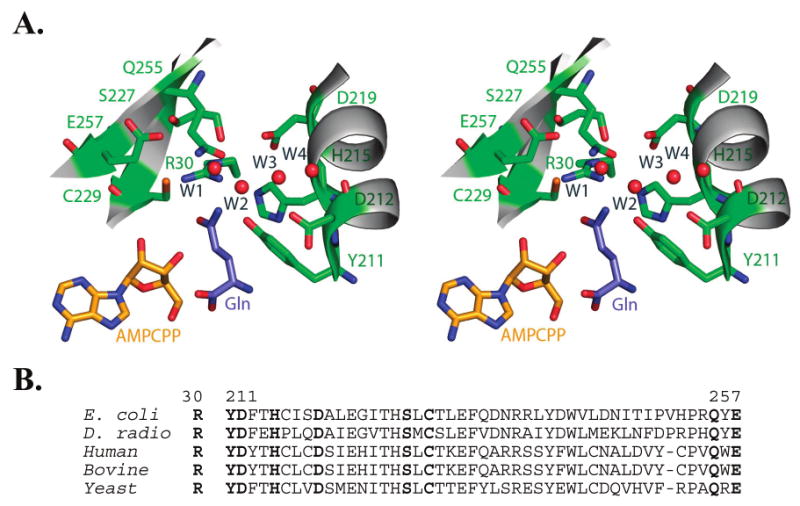
(A) Divergent stereoview of the glutamine binding pocket in E. coli GlnRS. Buried water molecules in the pocket are depicted as red spheres. Nomenclature for the waters follows Bullock et al. (12); see also Figures 3 and 5B herein. The phosphates of AMPCPP are disordered in this quaternary structure bound to substrate glutamine (12). The binding interactions made by the glutamine portion of a glutaminyl adenylate analogue are nearly identical (13). (B) Sequence alignment of selected GlnRS enzymes in the region corresponding to the glutamine binding pocket. Residues 211–260 comprise the second half of the catalytic Rossmann fold. Residues shown in panel A are highlighted in boldface.
