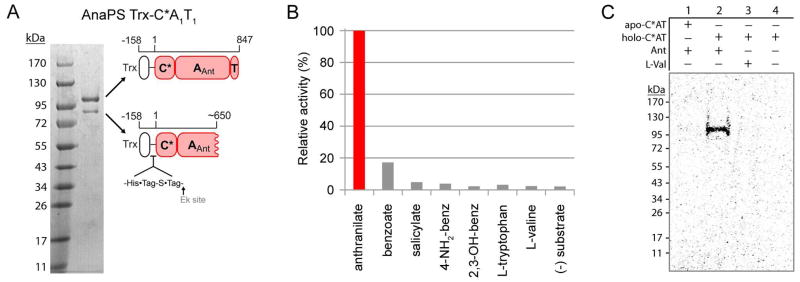
Figure 4.
Purification and characterization of AnaPS module 1. (A) Purity of the thioredoxin fusion protein of AnaPS module 1 (Trx-C*A1T1) post Ni-NTA chromatography and concentration-induced protein precipitation. Cartoon representation is used to indicate the identity and domain-composition of the two prominent bands present in the gel as determined by peptide mass fingerprinting. (B) ATP-[32P]PPi exchange assay data obtained for Trx-C*AT. 100% relative activity for anthranilate-dependent exchange corresponds to 6200 CPM. (C) Autoradiograph of SDS-PAGE gel illustrating the covalent loading of [carboxy-14C]anthranilate onto the holo-T-domain of Trx-C*AT.