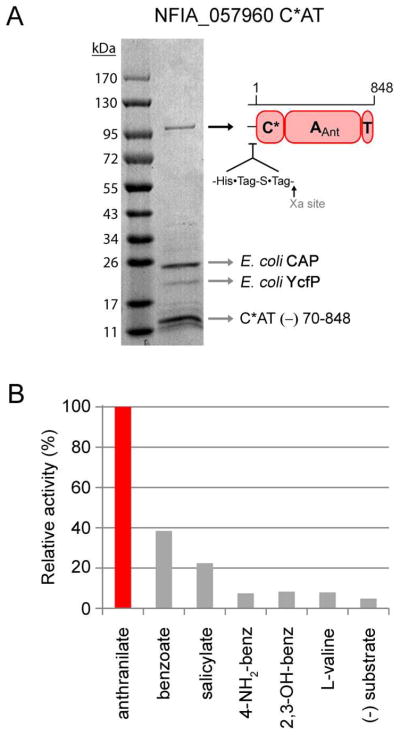
Figure 9.
Purification and characterization of NFIA_057960 module 1 C*AT. (A) Purity of the C*AT protein following Ni-NTA, anion-exchange, and gel-filtration chromatography. The identity of the ~100 kDa band as full-length C*AT protein, and the identities of the low-molecular-weight impurities indicated by arrows were determined by mass fingerprinting (CAP, catabolite gene activator protein; YcfP, conserved hypothetical protein; C*AT (−) 70-848, fragment of full-length protein missing residues ≈ 70-848). (B) Adenylation activity of C*AT with a panel of aryl-acids assessed by ATP-[32P]PPi exchange assay. L-valine is provided as a negative control.