Abstract
Similar modes of virus maturation have been observed in dsDNA bacteriophages and the structurally related herpes viruses and some type of maturation occur in most animal viruses. Recently a variety of biophysical studies of maturation intermediates of bacteriophages P22, λ, and HK97 have suggested an energy landscape that drives the transitions and structure-based mechanisms for its formation. Near-atomic resolution models of subunit tertiary structures in an early intermediate of bacteriophage HK97 maturation revealed a remarkable distortion of the secondary structures when compared to the mature particle. Scaffolding proteins may induce the distortion that is maintained by quaternary structure interactions following scaffold release, making the intermediate particle meta-stable.
Introduction
Large, multi-subunit, protein complexes initially assemble with weak interactions (~2–4kT) to avoid kinetic traps and to allow protein annealing for proper positioning[1,2]. Weak interactions are well suited for intracellular complexes since they assemble and disassemble in a transient fashion to activate correlated cellular functions. Virus particles, however, have a different agenda. Like all self-assembling systems they must initially form with weak interactions, however, a portion of their life cycle is extra-cellular requiring robust stability. The solution to these conflicting requirements is a staged assembly process in which the proper affinities exist for the efficient assembly of a particle followed by one or more subsequent steps in which the particle gains stability and infectivity. The initial particle is usually referred to as a Procapsid or Provirion and the subsequent events, encoded as a program in the Procapsid, are called maturation. Most animal viruses and bacteriophages mature, with varying degrees of conformational change in the capsid during the process. Nodaviruses undergo a subtle autocatalytic cleavage of the capsid subunits after they exit the cell and that stabilizes the particle and confers infectivity[3]. A similar event occurs in picornaviruses with the generation of VP4 and VP2 from precursor VP0 subunit[4]. Other non-enveloped viruses such as tetraviruses undergo dramatic reorganization of the particle during maturation, changing in size from ~500Å to ~400Å with the smaller particle undergoing autocatalytic cleavage as in the previous two viruses described[5]. This review focuses on maturation of isometric bacteriophages and the insights that have been gained about this process since the last review of the subject by Steven and colleagues[6].
Bacteriophage Maturation
Viruses examined here are bacteriophages λ[7], HK97[8] and P22[9]. These phages have near icosahedral capsids with T=7l quasi-equivalence that are formed by 11 pentamers and 60 hexamers of a single gene product. The basic fold of the subunits in each of these viruses is similar[7,10,11], containing an α/β “Axial” domain forming the 5-fold and quasi 6-fold interactions and an extended “Peripheral domain” between 3-fold axes formed by a 40A “Spine” helix, an extended “E-loop” and a compact “P-loop”. The P22 subunit is significantly larger with an additional domain, but the characteristic features are clearly visible in sub nanometer cryoEM structures. A dodecameric complex called the portal occupies the position of the 12th pentamer in the capsid. The portal is the site of attachment for the two dsDNA packaging proteins, collectively called terminase. Terminase appears comparable in most of the dsDNA bacteriophages and structural progress has been made with the small terminase of P22 and the large terminase of T4. An oligomer of 8 or 9 small subunits is believed to attach to the portal[12] while the large subunit is an ATPase enzyme that binds to dsDNA and the small terminase subunits providing the driving force for the packaging[13,14]. These bacteriophages undergo large scale reorganization of their capsid subunits during maturation and it occurs when roughly 30% of the dsDNA is packaged[15]. During maturation these particles change morphology from round to facetted, expand from ~500Å to 650Å and the shell thins from ~40Å to ~20Å. The path for assembly and maturation of P22 is shown in Figure 1. Closely similar events occur in HK97 and λ, however, these viruses have long tails for delivering the dsDNA into the cell in place of the tail machine complex.
Figure 1. The bacteriophage P22 assembly pathway.
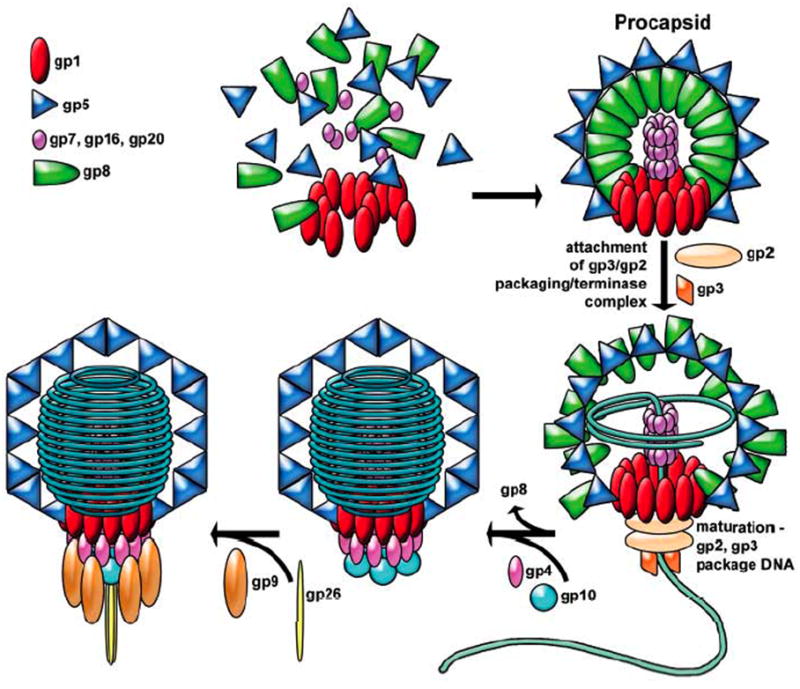
P22 assembles a protein precursor particle called a procapsid, which is the receptacle into which its 43.5 kbp DNA chromosome is packaged. P22 procapsid shells are built from two major components: 415 molecules of coat protein (gp5, the product of gene 5) arranged in a T = 7l icosahedral shell; and roughly 250 molecules of scaffolding protein (gp8) within the coat protein shell. In addition, four other proteins are present in the procapsid. A dodecamer of 84 kDa proteins (gp1) is present at a single unique icosahedral vertex. Six to twenty intravirion molecules of the products of genes 7, 16 and 20 are required for successful DNA injection into susceptible cells and are released from the virion during the injection process. As DNA is packaged, the thick procapsid shell expands from a radius of about 55 nm to a thinner, more angular shell 65 nm in diameter. Despite having a genome 41.7 kbp in length, P22 packages DNA until the capacity of the capsid is reached, which is at 43.5 kbp, a strategy referred to as head-full DNA packaging. Termination of packaging by cleavage of the concatemeric DNA is initiated not by sequence, but when the chromosome is at a defined packing density that is sensed by the portal protein. After DNA is packaged, the tail assembly is constructed by the sequential addition of multiple copies of four gene products (gp4, gp10, gp26 and gp9) to the vertex occupied by the portal ring. (taken from Lander et al 2006)
Scaffolding proteins set the stage
Initial assembly of bacteriophage capsids is guided by a “scaffolding” polypeptide that is either an independent gene product (P22 and λ), or is a portion of the capsid subunit that is removed by a virally encoded protease after formation of the procapsid (HK97). King, Casjens, and Prevelige extensively studied the 303 amino acid scaffold protein of P22 and its role in assembly. The results of this work, as well as studies of the herpes virus scaffold was comprehensively reviewed by Fane and Prevelige[16]. These studies, as they apply directly to particle assembly for P22, conclude that the scaffold protein promotes assembly by reducing the coat protein concentration required for assembly, guides the proper formation of the shell and recruits the portal and other minor proteins into the particle. Residues 238-303 interact with the capsid protein and have a helix loop helix motif. The overall protein is elongated and loosely folded. Less extensive studies were performed for the scaffolding proteins of bacteriophage lambda and herpes virus, but, to the extent investigated, they generally behave in a similar fashion and perform similar functions as the P22 scaffold. The delta domain of HK97 corresponds to residues 2-103 of the full length capsid subunit. Expressed on its own, the delta domain appears to be closely similar in physical properties to the independent scaffold proteins described (Huang and Johnson, unpublished). Here we propose an extension of the role of the scaffold protein, as suggested by crystallographic studies of HK97 intermediates, and propose that this role may be similar in other bacteriophages and possibly herpesvirus.
Initial expansion of P22 is exothermic
The transition that the P22 capsid undergoes during dsDNA packaging was studied for decades with a variety of biophysical methods. For the purpose of this review there are two papers published in the 1990s that stand out. Galisteo and King[17] studied the expansion of P22 in vitro with differential scanning calorimetry (DSC) and showed that the transition from procapsid to capsid was exothermic with the release of ~21kcal/mole of capsids. Steven [18] pointed out the novelty of the meta-stable state that must characterize the P22 procapsid in a “New and Notable” article in the journal, but where the energy came from was not addressed. Tuma et al[19] added a structural component to the energy storage question when they showed that significant change in deuterium accessibility occurred in the P22 subunits between Procapsid and Capsid. They interpreted this as the trapping of an intermediate tertiary structure during the folding and assembly of the P22 subunits and suggested that assembly trapped the folding intermediate and that the ground state was achieved in the mature capsid.
HK97 Assembly and Maturation
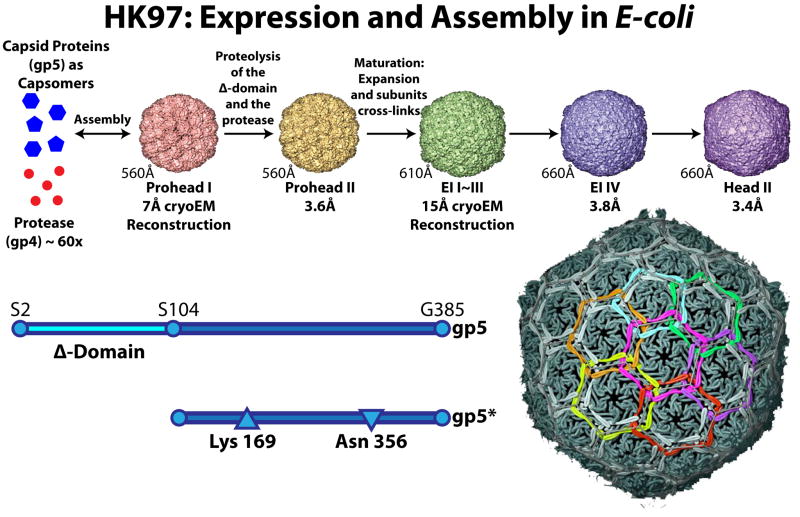
HK97 became an attractive subject for capsid maturation studies because virus-like particles could be made in the E. coli expression system and their maturation carried out in vitro[20]. The high resolution X-ray structure of the mature (Head II) particle was determined allowing an atomic structural interpretation of the biophysical data[21]. Figure 2 illustrates the maturation pathway mapped for these particles in a series of studies employing electron cryo-microscopy, X-ray crystallography, small angle X-ray scattering (SAXS) and biochemical methods[21–28]. Initial assembly into Prohead I occurs when the viral protease (gp4) and the capsid protein (gp5) are co-expressed in E. coli. If the protease is active, Prohead I has a short lifetime within the E. coli cells with the delta domain being immediately cleaved off and the protease auto-digesting into small polypeptides that diffuse out of the particle. The 17 MDa Prohead I is thus converted into the 13 MDa Prohead II which is the particle that is purified from the expression system and that can be used to study maturation in vitro. If an inactive protease is used in the co-expression, a virtually authentic version of Prohead I can be isolated, composed of full length gp5 and mutated gp4. These particles can be disassembled and reassembled in vitro. Expression of just the capsid protein results in assembled particles that can also be purified and disassembled into a mixture of hexameric and pentameric capsomers[27]. Thus, prior to the proteolysis assembly is a readily reversible process.
Figure 2. The HK97 virus-like particle assembly and maturation pathway that is followed when only the coat protein and protease are co-expressed in E. coli.
At the top are all the intermediates that have been characterized by crystallography and/or cryoEM. Below is shown the processing that occurs to the capsid protein, gp5, and the residues that form the auto-catalytic crosslink. At right is an enlargement of the final mature particle indicating that the cross-linked gp5 proteins mechanically chain-link the particle together. Each ring of the same color corresponds to 5 or 6 subunits chain-linked together by the isopeptide bond formed by the side chains of Asn 356 and Lys169. Assembly and Maturation: The capsid protein (gp5) immediately assembles into skewed hexamers and pentamers (generically called capsomers) shown in blue. The protease (gp4) is shown in red. The capsomers (60 hexamers and 12 pentamers) and approximately 60 copies of the protease co-assemble to form prohead I, a roughly spherical particle, ~51nm in diameter. The hexamers in this particle are not symmetric, but “skewed”, and correspond roughly to two shifted trapezoids (each composed of 3 subunits) related by 2-fold symmetry[23]. Under normal circumstances, prohead I is transient. The protease becomes active upon assembly and cleaves residues 2-103 (the delta domain) from all the gp5 subunits, creating gp5* as shown. The protease auto-digests and all of the fragments diffuse from openings in the capsid. This creates prohead II (~51nm) that is morphologically closely similar to prohead I (including the skewed hexamers and virtually the same diameter), however the mass of this particle is 13Mdalton, compared to 17Mdalton for prohead I. Prohead I can be stabilized for study by not expressing the protease, or co-expressing a mutant, inactive, protease. Either of these particles can be disassembled and reassembled under mild conditions[27] as indicated by the arrow from capsomers to prohead I. Prohead II is a meta-stable particle that can only transition to EI-1. Conditions that disassemble prohead I cause either nothing to happen or the transition to EI-1. There are many conditions that cause the transition, but dropping the pH from 7 to 4.0 was used for most of the in vitro studies of maturation. The transition to EI-1 (~56.0nm), triggered by the pH drop, has a half-life of ~3 minutes and is stochastic without populated intermediates. EI-1 hexamers are 6-fold symmetric and the particle is crosslink competent, with crosslink initiation commencing virtually immediately after this particle is formed. Approximately 60% of the crosslinks form before the morphology of EI-1 changes (EI-2,3 have the essentially the same morphology as EI-1 with increasing numbers of crosslinks) to EI-4. The process resembles a Brownian ratchet (a process by which thermal energy is captured by driving a process in only one direction) in which the loop containing Lys169 fluctuates until the covalent bond with Asn 356 forms, locking it down and incrementally raising the energy of the particle[31]. When a sufficient number form (~60%) the particle crosses the energy barrier and transitions to EI-4, again without populated intermediates, a round (~ 62.5nm), thin shelled particle eventually forming all but 60 crosslinks[38]. This particle is the end point at pH 4.0 and was studied by crystallography and cryoEM. These studies showed that subunits in the pentamers were not crosslinked and that they were dynamic fluctuating by 1.4nm along the 5-fold particle axes. Neutralizing EI-4 completes the crosslinks with pentamer subunits, forming the fully mature, ~65nm, faceted particle[31].
A series of in vitro studies with HK97 Prohead II maturation, initiated by lowering the pH to 4.5, revealed that the initial transition to Expansion Intermediate 1 (EI-1) occurred in a stochastic manner with no populated intermediates, a result that suggested the Prohead II particles were meta-stable, similar to the Prohead of P22[29]. A unique feature of the HK97-like capsids is the auto-catalytic formation of an isopeptide bond between Lys 169 on one subunit and Asn 356 on an adjacent subunit[30]. EI-1 is the first intermediate that is cross-link competent and covalent bonds begin forming between adjacent subunits immediately after expansion. When a critical number of bonds form (~60%) there is a sharp transition with an additional expansion and thinning of the shell[31]. Crosslinking continues until all but 60 have formed. This particle, referred to as EI-4, is stable and the crystal structure determined, revealing that only crosslinks involving subunits at the pentons were not formed[24]. When the pH was raised to 7 subunits at pentons formed crosslinks and the particle reached its mature, icosahedral shape. Each particle transition is remarkably cooperative with no populated intermediates detected.
We hypothesize that like P22 the first transition from Prohead II to EI-1 is exothermic. The second transition is reminiscent of a Brownian ratchet in which the crosslink formation drives the particle to a higher energy state[31]. Many crosslinks can be accommodated in a single quaternary structure (referred to as EI-II, III), but when the critical number form, the particle transitions to the EI-IV state. In this particle pentons are highly dynamic and the crosslinks involving these subunits do not form until the pH is raised to 7, presumably anchoring the pentons at the higher radius required for crosslink formation. Ross et al[32] characterized the stability associated with each of the maturation intermediates with DSC and established a free energy diagram relating them. The role of inter subunit interactions in driving the post-exothermic Brownian ratchet activity does not require subunit crosslinks, since non covalent interactions will drive the transition in mutants of HK97 that will not crosslink, although at a slower rate than crosslink competent particles[31]. Kang et al showed a similar effect in P22 where insertion of a cys residue at position 182 led to inter subunit disulfide formation that increased the rate of maturation[33]. Thus in P22 maturation may be driven by a non covalent Brownian ratchet.
Energy storage in meta-stable intermediate Particle
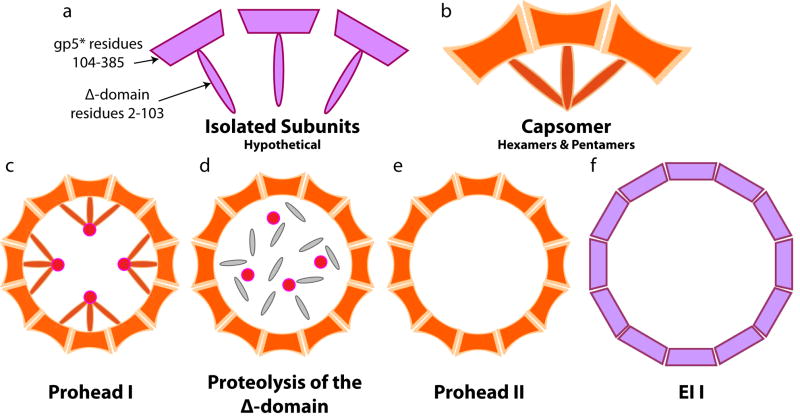
Tuma et al[19] provided a suggestion for the source of energy in the procapsids. The subunit fold changes observed with Raman spectroscopy during the P22 procapsid maturation lead to their proposal that a folding intermediate was trapped during particle assembly and when the particle was perturbed, subunit folding proceeded to the “ground state”, making the transition exothermic. Gertsman et al[22] provided three dimensional structural evidence for such a model when they determined the crystal structure of the prohead II particle of HK97. They found that, while the subunit fold in the procapsid state was recognizable as the HK97 fold observed in the mature Head II particle, the tertiary structure was distorted with the long spine helix partially unfolded and bent as well as a twist of up to 35° about three strands of beta structure. There were significant differences in the degree of distortion among the 7 subunits in the icosahedral aymmetric unit and it appeared dependent on the location of the subunit in the quaternary structure. They proposed that the ubiquitous presence of skewed hexamers in procapsids reflected a trapped folding intermediate in procapsids. Their hypothesis differed from that of Tuma et al in that their folding intermediate was induced by interaction of the delta domains. Direct evidence for the role of delta domains in distorting the quaternary structure of the capsid came from DSC of Prohead I [23]. Conway et al showed, with cryoEM, that a reversible change in thermal absorbance at 53° caused partial particle expansion and a change in hexamer configuration from skewed to symmetric as well as a change in density only for the delta domains associated with the hexamers. The observation suggests that when delta domain interactions were perturbed by heat, it allowed the hexamers to become symmetric. The generalized proposal was that scaffold proteins interact with viral subunits during assembly, distort the fold and the distortion is trapped by the quaternary structure formation when the scaffold is released. Delta domains are different from other scaffold proteins only in the sense that they have to be removed by proteolysis rather than being released spontaneously following assembly. Figure 3 provides a schematic representation of the hypothesis for the initial HK97 maturation event.
Figure 3. A cartoon depicting the working hypothesis for creating an energy landscape that makes HK97 maturation exothermic.
a. Hypothetical, full length, monomeric, gp5 subunits with the Δ-domain depicted as an extension away from the gp5* digestion product. Without Δ-domain interactions the model conjectures that the gp5* portion of the subunit is undistorted. b. Upon expression the Δ-domains of the gp5 subunits immediately interact with each other forming capsomers with skewed hexamers and distorted subunit tertiary structures as observed in the crystal structure of prohead II. The model posits that interactions between the Δ-domains distort the tertiary structures and balance the energy required for the distortion. c. Capsomers assemble to form prohead I with quaternary and tertiary structure very similar to that seen in prohead II and also package the protease, depicted a blue circles in the cartoon. If proteolysis is inhibited, prohead I can be disassembled under mild conditions and reassembled. d. Following proteolysis, the Δ-domains are removed and the quaternary structure traps the tertiary structure distortion caused by Δ-domain interactions. e. The prohead II particle is now metastable with the energy for the distortion of the tertiary structure trapped by the quaternary structure in the absence of Δ-domain interactions. f. When the metastable particle is perturbed from its local energy minima, the distorted subunits reach their “ground state” structure with the release of energy. This occurs during the transition to intermediate EI-1.
Are In vitro studies of virus maturation relevant?
How do the in vitro studies of HK97 maturation relate to the events as they occur in the cell? As discussed, maturation is normally initiated by packaging about 30% of the dsDNA. The Prohead II crystal structure revealed that the internal portion of the capsid is dominated by acidic residues[22], creating an excess of negative charge and there is strong evidence that this is also the case for P22[34]. We believe that the electrostatic effect of negatively charged dsDNA encountering the internal negative surface initiates a perturbation that results in the formation of EI-I. As more dsDNA is packaged, crosslinking initiates, making the EI-II, III particle. When sufficient crosslinks have occurred the particle transitions to EI-IV that is at neutral pH in the cell and thus probably transitions directly to the fully mature particle Head II. In vivo the process is probably faster than in vitro because the physical pressure of the packaged dsDNA most likely adds an additional driving force to the transitions[35].
Conclusions
Capsid maturation is an accessible natural example of a nano machine. With only two gene products HK97 creates an energy landscape that populates a variety of structurally distinct intermediates, arriving at a remarkably robust particle stabilized by chain-linked rings of covalently joined proteins. This complex and precise program is entirely encoded in the structure of the initial assembly product, driven to a meta-stable state by proteolysis and triggered into a cascade of spontaneous expansion and ratcheted maturation through the packaging of dsDNA. While the details differ, it is likely that maturation of bacteriophages lambda and P22, as well as the structurally-related herpesvirus are driven by similar mechanisms. All have scaffolding proteins that are transiently associated with the viral subunits and all undergo dramatic expansions. Lambda gains its stability by binding an accessory protein called gpD instead of forming crosslinks[7]. Binding of gpD only occurs following initial particle expansion and it may be that binding of gpD performs the role of crosslinks, in both stabilizing the particle and driving the final stages of maturation through its attachment. P22 has an additional domain in its subunit that appears critical for the robust stability of the mature particle[10]. Like HK97, Herpes virus requires a virally encoded protease to release its scaffold[36] and the capsid is more complex than the phage, incorporating multiple types of stabilizing proteins in its T=16 surface lattice[37]. Experimental studies of the transitions between the stable intermediate particles are the next challenge in virus maturation. These studies require single particle investigation where fluorescent reporters, that are sensitive to the transients, may allow the unpopulated intermediates to be observed.
Acknowledgments
The HK97 experiments co-authored from our laboratory were a collaborative effort with the laboratories of Robert Duda, James Conway, Alasdair Steven and Roger Hendrix. The exceptional generosity, expertise and intellectual acumen of these collaborators, as well as the pleasure of their company, are deeply appreciated and heartily acknowledged. Major contributors to the crystallographic, small angle X-ray scattering (SAXS), cryoEM and biochemical studies in the author’s laboratory were Ilya Gertsman, Kelly Lee, Lu Gan, Rick Huang, Gabriel Lander and William Wycoff. Their skill and enthusiasm have made work on this system a source of constant enjoyment. Hiro Tsuruta (Stanford Synchrotron Radiation Lightsource) has been a major contributor to all of our SAXS studies and it is a pleasure to acknowledge his skill, effort and good-natured enthusiasm for this work. I am grateful to Gabriel Lander for the preparation of Figure 1 and to Rick Huang for the preparation of Figures 2 and 3. National Institutes of Health grant R01-AI040101 supported the HK97 work in the author’s laboratory.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
• of special interest
•• of outstanding interest
- 1.Caspar DL. Movement and self-control in protein assemblies. Quasi-equivalence revisited. Biophys J. 1980;32:103–138. doi: 10.1016/S0006-3495(80)84929-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2 .Katen S, Zlotnick A. The thermodynamics of virus capsid assembly. In: Johnson ML, Holt JM, Ackers GK, editors. Methods in Enzymology: Biothermodynamics, part A. Vol. 395. Academic Press; 2009. pp. 417–455. An excellent review of the thermodynamics and kinetics of assembly of icosahedral viruses that explicitly addresses the role of weak subunit interactions in successful particle assembly. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schneemann A, Zhong W, Gallagher TM, Rueckert RR. Maturation cleavage required for infectivity of a nodavirus. J Virol. 1992;66:6728–6734. doi: 10.1128/jvi.66.11.6728-6734.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Larsen GR, Anderson CW, Dorner AJ, Semler BL, Wimmer E. Cleavage sites within the poliovirus capsid protein precursors. J Virol. 1982;41:340–344. doi: 10.1128/jvi.41.1.340-344.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Matsui T, Lander G, Johnson JE. Characterization of large conformational changes and autoproteolysis in the maturation of a T=4 virus capsid. J Virol. 2009;83:1126–1134. doi: 10.1128/JVI.01859-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Steven AC, Heymann JB, Cheng N, Trus BL, Conway JF. Virus maturation: dynamics and mechanism of a stabilizing structural transition that leads to infectivity. Curr Opin Struct Biol. 2005;15:227–236. doi: 10.1016/j.sbi.2005.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7 .Lander GC, Evilevitch A, Jeembaeva M, Potter CS, Carragher B, Johnson JE. Bacteriophage lambda stabilization by auxiliary protein gpD: timing, location, and mechanism of attachment determined by cryo-EM. Structure. 2008;16:1399–1406. doi: 10.1016/j.str.2008.05.016. The decoration protein of phage λ (gpD) was shown to interact at the trimer contacts of the virus capsid with the N-terminii of gpD adding a strand to a β-sheet of the capsid protein. gpD clearly plays an equivalent role for λ as the crosslinks play for HK97. A lower resolution structure of the λ procapsid accomodated the X-ray model of a protein with high homology to the λ subunit. This subunit had a significantly different tertiary structure from that modeled in the mature particle suggesting that, as in HK97 there is a tertiary structure change between procapsid and capsid. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wikoff WR, Conway JF, Tang J, Lee KK, Gan L, Cheng N, Duda RL, Hendrix RW, Steven AC, Johnson JE. Time-resolved molecular dynamics of bacteriophage HK97 capsid maturation interpreted by electron cryo-microscopy and X-ray crystallography. J Struct Biol. 2006;153:300–306. doi: 10.1016/j.jsb.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 9.Lander GC, Tang L, Casjens SR, Gilcrease EB, Prevelige P, Poliakov A, Potter CS, Carragher B, Johnson JE. The structure of an infectious P22 virion shows the signal for headful DNA packaging. Science. 2006;312:1791–1795. doi: 10.1126/science.1127981. [DOI] [PubMed] [Google Scholar]
- 10.Jiang W, Li Z, Zhang Z, Baker ML, Prevelige PE, Jr, Chiu W. Coat protein fold and maturation transition of bacteriophage P22 seen at subnanometer resolutions. Nat Struct Biol. 2003;10:131–135. doi: 10.1038/nsb891. [DOI] [PubMed] [Google Scholar]
- 11.Wikoff WR, Liljas L, Duda RL, Tsuruta H, Hendrix RW, Johnson JE. Topologically linked protein rings in the bacteriophage HK97 capsid. Science. 2000;289:2129–2133. doi: 10.1126/science.289.5487.2129. [DOI] [PubMed] [Google Scholar]
- 12 .Nemecek D, Lander GC, Johnson JE, Casjens SR, Thomas JGJ. Assembly Architecture and DNA Binding of the Bacteriophage P22 Terminase Small Subunit. J Mol Biol. 2008;383:494–501. doi: 10.1016/j.jmb.2008.08.050. A low resolution structure of the small terminase subunit showing its quaternary structure and suggesting a model for its interaction with the portal. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13 .Sun S, Kondabagil K, Draper B, Alam TI, Bowman VD, Zhang Z, Hegde S, Fokine A, Rossmann MG, Rao VB. The structure of the phage T4 DNA packaging motor suggests a mechanism dependent on electrostatic forces. Cell. 2008;135:1251–1262. doi: 10.1016/j.cell.2008.11.015. A detailed model for the DNA packaging pump that incorporates large conformational changes with the hydrolysis of ATP. The work provides the first insight into the mechanism of the strongest biological motor yet characterized. [DOI] [PubMed] [Google Scholar]
- 14.Sun S, Kondabagil K, Gentz PM, Rossmann MG, Rao VB. The structure of the ATPase that powers DNA packaging into bacteriophage T4 procapsids. Mol Cell. 2007;25:943–949. doi: 10.1016/j.molcel.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 15 ..Fuller DN, Raymer DM, Rickgauer JP, Robertson RM, Catalano CE, Anderson DL, Grimes S, Smith DE. Measurements of single DNA molecule packaging dynamics in bacteriophage lambda reveal high forces, high motor processivity, and capsid transformations. J Mol Biol. 2007;373:1113–1122. doi: 10.1016/j.jmb.2007.09.011. A direct observaton of virus maturation observed with single particle methods. A change in resistance to DNA packaging occurs consistently at ~30% of the DNA packaged and is suggested to correspond to capsid expansion. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fane BA, Prevelige PE., Jr Mechanism of scaffolding-assisted viral assembly. Adv Protein Chem. 2003;64:259–299. doi: 10.1016/s0065-3233(03)01007-6. [DOI] [PubMed] [Google Scholar]
- 17.Galisteo ML, King J. Conformational transformations in the protein lattice of phage P22 procapsids. Biophys J. 1993;65:227–235. doi: 10.1016/S0006-3495(93)81073-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stevens A. Conformational Change, an Alternative Energy Source?: Exothermic Phase Transition in Phage Capsid Maturation. Biophys J. 1993;65:5–6. doi: 10.1016/S0006-3495(93)81022-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tuma R, Prevelige PE, Jr, Thomas GJ., Jr Mechanism of capsid maturation in a double-stranded DNA virus. Proc Natl Acad Sci U S A. 1998;95:9885–9890. doi: 10.1073/pnas.95.17.9885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hendrix RW, Duda RL. Bacteriophage HK97 head assembly: a protein ballet. Adv Virus Res. 1998;50:235–288. doi: 10.1016/s0065-3527(08)60810-6. [DOI] [PubMed] [Google Scholar]
- 21.Wikoff W, Liljas L, Duda R, Tsuruta H, Hendrix R, Johnson J. Topologically linked protein rings in the bacteriophage HK97 capsid. Science. 2000;289:2129–2133. doi: 10.1126/science.289.5487.2129. [DOI] [PubMed] [Google Scholar]
- 22 ..Gertsman I, Gan L, Guttman M, Lee K, Speir JA, Duda RL, Hendrix RW, Komives EA, Johnson JE. An unexpected twist in viral capsid maturation. Nature. 2009;458:646–651. doi: 10.1038/nature07686. The first near-atomic resolution X-ray structure of a dsDNA bacteriophage procapsid. It showed extensive distortion of the tertiary structure of the HK97 subunit when compared to the mature HK97 and suggested the mechanism of meta stability presented here. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23 ..Conway JF, Cheng N, Ross PD, Hendrix RW, Duda RL, Steven AC. A thermally induced phase transition in a viral capsid transforms the hexamers, leaving the pentamers unchanged. J Struct Biol. 2007;158:224–232. doi: 10.1016/j.jsb.2006.11.006. Direct evidence that the δ-domains cause the skewing of the hexamers. A transition observed in the differential scanning calorimetry experiment was shown by cryoEM to correspond to an expansion of the particle and symmetrization of the hexamers. The transition was reversible, showing that δ-domains controlled this and prevented the particle from maturing to prohead II. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24 .Gan L, Speir JA, Conway JF, Lander G, Cheng N, Firek BA, Hendrix RW, Duda RL, Liljas L, Johnson JE. Capsid conformational sampling in HK97 maturation visualized by X-ray crystallography and cryo-EM. Structure. 2006;14:1655–1665. doi: 10.1016/j.str.2006.09.006. Combined X-ray crystallography and cryoEM studies of EI-4 showed that the pentamer subunits did not form crosslinks in this intermediate and that the pentamers were highly dynamic with a 1.4nm piston-like motion along the 5-fold axis. [DOI] [PubMed] [Google Scholar]
- 25.Lee KK, Tsuruta H, Hendrix RW, Duda RL, Johnson JE. Cooperative reorganization of a 420 subunit virus capsid. J Mol Biol. 2005;352:723–735. doi: 10.1016/j.jmb.2005.07.024. [DOI] [PubMed] [Google Scholar]
- 26.Lata R, Conway JF, Cheng N, Duda RL, Hendrix RW, Wikoff WR, Johnson JE, Tsuruta H, Steven AC. Maturation dynamics of a viral capsid: visualization of transitional intermediate states. Cell. 2000;100:253–263. doi: 10.1016/s0092-8674(00)81563-9. [DOI] [PubMed] [Google Scholar]
- 27.Duda RL, Hempel J, Michel H, Shabanowitz J, Hunt D, Hendrix RW. Structural transitions during bacteriophage HK97 head assembly. J Mol Biol. 1995;247:618–635. doi: 10.1006/jmbi.1995.0168. [DOI] [PubMed] [Google Scholar]
- 28.Conway JF, Duda RL, Cheng N, Hendrix RW, Steven AC. Proteolytic and conformational control of virus capsid maturation: the bacteriophage HK97 system. J Mol Biol. 1995;253:86–99. doi: 10.1006/jmbi.1995.0538. [DOI] [PubMed] [Google Scholar]
- 29.Lee KK, Gan L, Tsuruta H, Hendrix RW, Duda RL, Johnson JE. Evidence that a local refolding event triggers maturation of HK97 bacteriophage capsid. J Mol Biol. 2004;340:419–433. doi: 10.1016/j.jmb.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 30 ..Dierkes LE, Peebles CL, Firek BA, Hendrix RW, Duda RL. Mutational analysis of a conserved glutamic acid required for self-catalyzed cross-linking of bacteriophage HK97 capsids. J Virol. 2009;83:2088–2098. doi: 10.1128/JVI.02000-08. A biochemical and mutagenesis study showing the role of E363 in catalyzing the crosslink reaction of HK97. A detailed mechanisim for the auto-catalysis was proposed based on these studies as well as establishing the role of E363 on the kinetics of the transition and the morphology of assembly. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31 .Lee KK, Gan L, Tsuruta HCM, Conway JF, Duda RL, Hendrix RW, Steven AC, Johnson JE. Virus capsid expansion driven by the capture of mobile surface loops. Structure. 2008;16:1491–1502. doi: 10.1016/j.str.2008.06.014. A correlated biochemical and SAXS study showing that in vitro particle expansion from EI-1 morphology to EI-4 occurs when ~60% of the crosslinks form suggesting that this stage of expansion is driven by a Brownian ratchet. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32 .Ross PD, Conway JF, Cheng N, Dierkes L, Firek BA, Hendrix RW, Steven AC, Duda RL. A free energy cascade with locks drives assembly and maturation of bacteriophage HK97 capsid. J Mol Biol. 2006;364:512–525. doi: 10.1016/j.jmb.2006.08.048. A calorimetry study that characterized the thermal stability of the intermediates in HK97 expansion and used the data to set up a free energy relationship between the intermediates. The study also emphasized the role of the δ domain as a lock in controlling the timing of expansion. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33 .Kang S, Hawkridge AM, Johnson KL, Muddiman DC, Prevelige PE., Jr Identification of subunit-subunit interactions in bacteriophage P22 procapsids by chemical cross-linking and mass spectrometry. J Proteome Res. 2006;5:370–377. doi: 10.1021/pr050356f. Crosslink formation in the cys 182 mutant of the P22 subunit increases the rate of maturation, suggesting that a step in maturation may involve a non covalent Brownian ratchet similar to the crosslink driven Brownian ratchet in HK97. [DOI] [PubMed] [Google Scholar]
- 34.Parker MH, Prevelige PE., Jr Electrostatic interactions drive scaffolding/coat protein binding and procapsid maturation in bacteriophage P22. Virology. 1998;250:337–349. doi: 10.1006/viro.1998.9386. [DOI] [PubMed] [Google Scholar]
- 35 .Duda RL, Ross PD, Cheng N, Firek BA, Hendrix RW, Conway JF, Steven AC. Structure and energetics of encapsidated DNA in bacteriophage HK97 studied by scanning calorimetry and cryo-electron microscopy. J Mol Biol. 2009;391:471–483. doi: 10.1016/j.jmb.2009.06.035. An extension of the study in reference 32 showing the role of DNA on the thermal stability of virions. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu F, Roizman B. Characterization of the protease and other products of amino-terminus-proximal cleavage of the herpes simplex virus 1 UL26 protein. J Virol. 1993;67:1300–1309. doi: 10.1128/jvi.67.3.1300-1309.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhou ZH, Dougherty M, Jakana J, He J, Rixon FJ, Chiu W. Seeing the herpesvirus capsid at 8.5 A. Science. 2000;288:877–880. doi: 10.1126/science.288.5467.877. [DOI] [PubMed] [Google Scholar]
- 38.Gan L, Conway JF, Firek BA, Cheng N, Hendrix RW, Steven AC, Johnson JE, Duda RL. Control of crosslinking by quaternary structure changes during bacteriophage HK97 maturation. Mol Cell. 2004;14:559–569. doi: 10.1016/j.molcel.2004.05.015. [DOI] [PubMed] [Google Scholar]