Figure 1. The bacteriophage P22 assembly pathway.
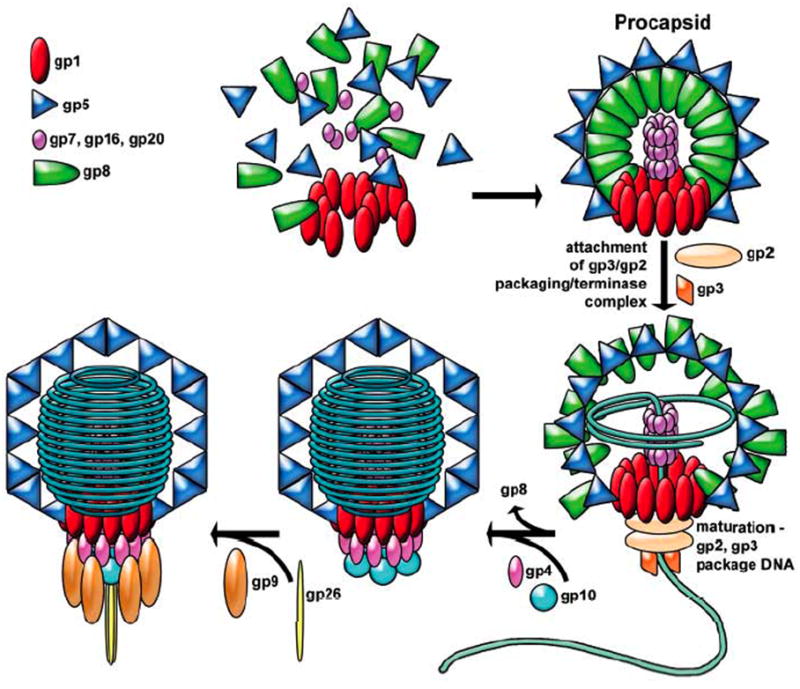
P22 assembles a protein precursor particle called a procapsid, which is the receptacle into which its 43.5 kbp DNA chromosome is packaged. P22 procapsid shells are built from two major components: 415 molecules of coat protein (gp5, the product of gene 5) arranged in a T = 7l icosahedral shell; and roughly 250 molecules of scaffolding protein (gp8) within the coat protein shell. In addition, four other proteins are present in the procapsid. A dodecamer of 84 kDa proteins (gp1) is present at a single unique icosahedral vertex. Six to twenty intravirion molecules of the products of genes 7, 16 and 20 are required for successful DNA injection into susceptible cells and are released from the virion during the injection process. As DNA is packaged, the thick procapsid shell expands from a radius of about 55 nm to a thinner, more angular shell 65 nm in diameter. Despite having a genome 41.7 kbp in length, P22 packages DNA until the capacity of the capsid is reached, which is at 43.5 kbp, a strategy referred to as head-full DNA packaging. Termination of packaging by cleavage of the concatemeric DNA is initiated not by sequence, but when the chromosome is at a defined packing density that is sensed by the portal protein. After DNA is packaged, the tail assembly is constructed by the sequential addition of multiple copies of four gene products (gp4, gp10, gp26 and gp9) to the vertex occupied by the portal ring. (taken from Lander et al 2006)
