Fig 2.
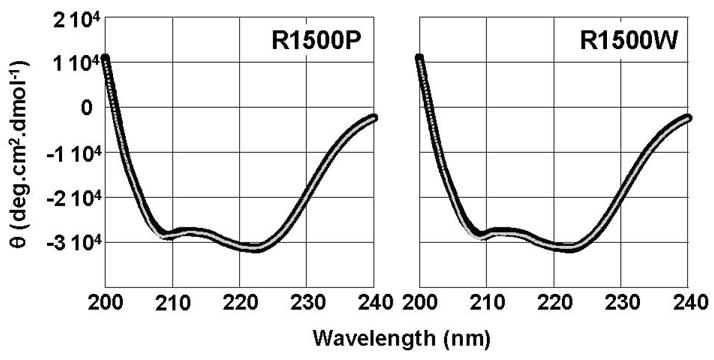
R1500P and R1500W mutations do not detectibly alter the secondary structure of LMM. Far-UV CD spectra of WT (black) and mutant (gray) LMM were obtained from 250 nm – 200 nm at 4 °C. WT LMM has nearly identical secondary structure profiles to R1500P and R1500W LMM. All proteins display canonical α-helical spectra with characteristic minima at 208 nm and 222 nm, and the calculated percentage of α-helix is similar for all three proteins.
