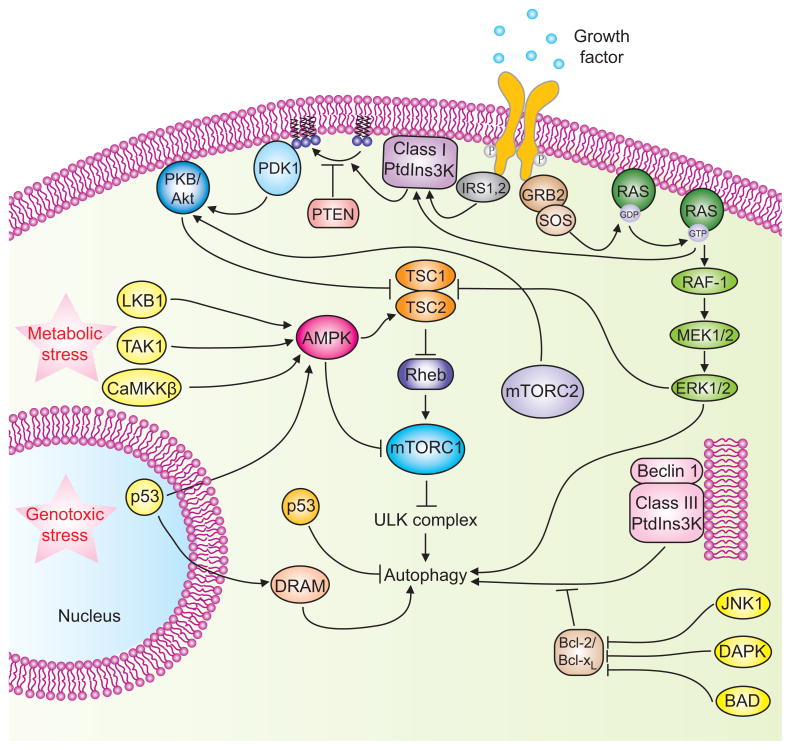
Figure 2. Signaling cascades involved in the regulation of mammalian autophagy.
Autophagy is regulated by a complex signaling network of various stimulatory (arrowheads) and inhibitory (bars) inputs. Activation of growth factor receptors stimulates the class I PtdIns3K complex and small GTPase Ras, which leads to activation of the PtdIns3K-PKB-mTORC1 pathway and the Raf-1-MEK1/2-ERK1/2 pathway, respectively. PKB and ERK1/2 phosphorylate and inhibit the GTPase-activating protein complex TSC1/TSC2, leading to the stabilization of Rheb-GTPase, which, in turn, activates mTORC1, causing inhibition of autophagy. Activated ERK1/2 also stimulates autophagy. mTORC2 inhibits autophagy through the phosphorylation and activation of PKB. Metabolic stress, such as high AMP/ATP ratios resulting from energy depletion, or an increase in the cytosolic free Ca2+ concentration or cytokines, cause the AMP-activated protein kinase (AMPK) to be phosphorylated and activated by LKB1, CaMKKβ and TAK1, respectively. AMPK phosphorylates and activates TSC1/TSC2, leading to inactivation of mTORC1 and autophagy induction. Genotoxic and oncogenic stresses result in nuclear p53 stabilization and activation, which stimulates autophagy through activation of AMPK or upregulation of DRAM. In contrast, cytosolic p53 has an inhibitory effect on autophagy. Anti-apoptotic proteins, Bcl-2 or Bcl-XL, associate with Beclin 1 and inhibit the Beclin 1-associated class III PtdIns3K complex, causing inhibition of autophagy. For additional details, see the main text.