Figure 1. mTOR signaling pathway.
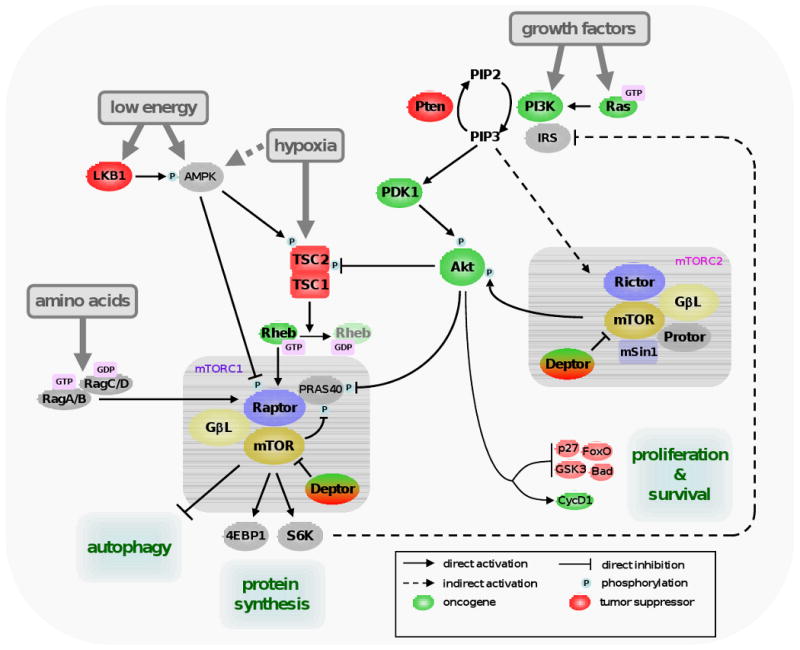
One branch of mTORC1 activation is mediated by the class I phosphoinositide-3 kinase (PI3K), Akt (also known as Pkb) and the tuberous sclerosis complex (TSC). TSC is formed by TSC1 and TSC2, and inhibits a direct activator of mTORC1, the GTPase Ras-homolog enriched in brain (Rheb) by hydrolyzing its GTP into GDP [52]. TSC2 is activated by phosphorylation by AMP-activated protein kinase (AMPK) [53], which is directly activated by a high AMP vs. ATP ratio. AMPK also directly phosphorylates and inactivates Raptor, so it inhibits mTORC1 by TSC-dependent and -independent manners [54]. The activity of AMPK is regulated by phosphorylation by the tumor suppressor LKB1. This protein, like TSC1/2, was found mutated in the germline of patients with different hamartomatous syndromes [55,56]. Akt is a serine/threonine kinase and an important player in regulating mTORC1 activity. Akt positively regulates mTORC1 by acting at different levels. First, Akt inactivates TSC1/2 by phosphorylating TSC2 [57]. Second, Akt inhibits Pras40, negative regulator of mTORC1 that counteracts Rheb function [6,7]. Akt is activated by PI3K, which responds to a variety of growth factors. When activated by insulin or insulin-like growth factors (IGFs), as well as other growth factors, class I PI3K catalyzes the formation of the lipidic second messenger phosphoinositide 3,4,5 tri-phosphate (PIP3) from the bi-phosphate form PIP2. PIP3 triggers the relocation of Akt to the inner surface of the plasma membrane, where it is activated by phosphoinositide-dependent kinase 1 (PDK1) and transduces the signal as described above. Opposing Akt function is the tumor suppressor Phosphatase and Tensin homolog deleted on chromosome ten (Pten), a lipid phosphatase that converts of PIP3 to PIP2, thus shutting off signaling from PI3K. Pten deficiency causes a series of hamartomatous syndromes collectively classified as PTEN hamartoma tumour syndrome (reviewed in [58]). Amino acids activate mTORC1 by an independent route mediated by the Rag family of proteins. The activation of mTORC2 is not well-understood, but this complex directly activates Akt (and Akt-related kinases) by phosphorylation. Akt, in addition, regulates many proteins involved in cell survival and cell cycle progression.
