Abstract
The role of axial structures, especially the notochord, in metanephric kidney development has not been directly examined. Here, we showed that disruption of the notochord and floor plate by diphtheria toxin (DTA)-mediated cell ablation did not disrupt nephrogenesis, but resulted in kidney fusions, resembling horseshoe kidneys in humans. Axial disruptions led to more medially positioned metanephric mesenchyme (MM) in midgestation. However, neither axial disruption nor the ensuing positional shift of the MM affected the formation of nephrons and other structures within the kidney. Response to Shh signaling was greatly reduced in midline cell populations in the mutants. To further ascertain the molecular mechanism underlying these abnormalities, we specifically inactivated Shh in the notochord and floor plate. We found that depleting the axial source of Shh was sufficient to cause kidney fusion, even in the presence of the notochord. These results suggested that the notochord is dispensable for nephrogenesis but required for the correct positioning of the metanephric kidney. Axial Shh signal appears to be critical in conferring the effects of axial structures on kidney positioning along the mediolateral axis. These studies also provide insights into the pathogenesis of horseshoe kidneys and how congenital kidney defects can be caused by signals outside the renal primordia.
Keywords: Kidney development, Horseshoe kidney, Notochord, Sonic Hedgehog, Diphtheria toxin, Ureteric bud, Metanephric mesenchyme, Intermediate mesoderm, Wolffian duct, Mediolateral positioning
Introduction
The kidney derives from the intermediate mesoderm (IM) that is a narrow strip of mesodermal cells sandwiched between the paraxial mesoderm and the lateral plate mesoderm. During embryogenesis, three sets of nephric structures (pronephros, mesonephros, and metanephros) emerge from the nephric cord within the IM in succession from an anterior to posterior direction. In mammals, the metanephric kidney is the definitive kidney. Some components of the transient mesonephric kidney contribute to the development of the metanephric kidney. The pair of epithelial ducts connecting the mesonephros to the cloaca are called the mesonephric duct or Wolffian duct (WD). During mid-gestation, the ureteric bud (UB) emerges from the WD (at about E10.5 in mice) and invades the metanephric mesenchyme (MM) within the IM. MM expresses a combination of factors important for its differentiation and the induction of the UB from the WD (Dressler, 2006). Lim1, Pax2, and Pax8, expressed early in both the WD and the MM, are indispensible for renal development. A delicate regulatory network modulates the activity of the receptor tyrosine kinase Ret and its ligand Gdnf, ensuring that only one UB emerges from the correct axial location of the WD (Schedl, 2007). Once inside the MM, the UB undergoes extensive branching morphogenesis. The tips of the UB induce the mesenchymal-epithelial transformation of the adjacent MM to form functional segments of the nephron. The UB later becomes the ureteral epithelium and the collecting ducts within the kidney (Dressler, 2006).
The notochord is a flexible, rod-shaped structure composed of cells derived from the mesoderm and defines the primitive axis in embryos of all chordates. It persists as the axial structural support throughout life in lower vertebrates, but is replaced by the vertebral column in higher vertebrates. In higher vertebrates, the notochord is not just a transient structural support for the embryos, but is also an important organizer for embryogenesis. The notochord runs along the anterior-posterior (AP) midline of the embryo and provides cues to the surrounding tissues for positioning and fate determination. It is well established that the notochord is essential for the formation of the floor plate of the neural tube in which the gradient generated by the Hedgehog (Hh) proteins, particularly Sonic Hedgehog (Shh), from the notochord is instrumental (Placzek, 1995; Stemple, 2005). In addition, Bmp inhibitors Noggin and Chordin from the notochord have also been thought to have key roles in guiding the development of surrounding structures (Stemple, 2005). Previous studies have indicated that signals with unknown nature from axial and paraxial tissues are involved in patterning of the intermediate mesoderm (Barak et al., 2005; James and Schultheiss, 2003; Mauch et al., 2000). It is also suspected that the renal agenesis in Danforth’s short tail (Sd) homozygous mutants is caused by notochord degeneration (Maatman et al., 1997). However, the role of the notochord and floor plate on metanephric kidney development has not been directly examined. It is still unclear what the key signals are for determining the position of the MM and the exact tissues where these key signals come from.
In this study, we examine how midline signals from axial structures, especially the notochord and floor plate, influence metanephric kidney development by using Diphtheria Toxin (DTA)-mediated cell ablation to disrupt the notochord and floor plate of the neural tube. To our surprise, we found that, instead of renal agenesis, disruption of the notochord and floor plate results in the formation of horseshoe kidneys. Further analyses indicate that Shh signaling is particularly important for the axial effects on mediolateral positioning of the kidneys.
Materials and Methods
Mouse (Mus musculus) strains and sample collection
All animal studies have been approved by IACUC (Institutional Animal Care and Use Committee) at Washington University School of Medicine and conducted as per the NIH guidelines. The NFP (Notochord and floor plate)-Cre transgene uses a Foxa2 enhancer element to drive Cre expression in the notochord and floor plate (Kumar et al., 2007). Mice carrying this transgene were crossed with mice carrying the ROSADTA allele (Wu et al., 2006) to produce NFP-Cre;ROSADTA/+ mice that would result in DTA-mediated apoptosis in notochord and floor plate. In Cre-positive cells, Cre-mediated loxP recombination removes a transcriptional “STOP” cassette in the ROSADTA allele, leading to the expression of DTA under the control of the ROSA promoter (Fig. 1C) (Jia et al., 2008; Wu et al., 2006). DTA can cause translational arrest and apoptosis through its inhibition of the eukaryotic elongation factor 2 (Wu et al., 2006). Some of the NFP-Cre;ROSADTA/+ mice survived to adulthood and were used to generate NFP-Cre;ROSADTA/DTA mice. Littermates with no cell ablation were used as controls. We also combined the NFP-Cre transgene and the ShhloxP allele (Yu et al., 2002) to produce NFP-Cre;ShhloxP/+ mice. Mice carrying ShhloxP were obtained from the Jackson laboratory (Bar Harbor, Maine). Further crosses produced the NFP-Cre;ShhloxP/loxP mice with homozygous deletion of Shh in cells expressing the NFP-Cre transgene. Littermates with no homozygous Shh deletion in any cells were used as controls. The ROSALacZ allele (Soriano, 1999) was used as a reporter to fate map cells expressing Cre. Mice carrying Gli1lacZ were obtained from the Jackson laboratory (Bar Harbor, Maine) and were crossed with NC-DTA mutants to reveal cells responding to Shh signaling.
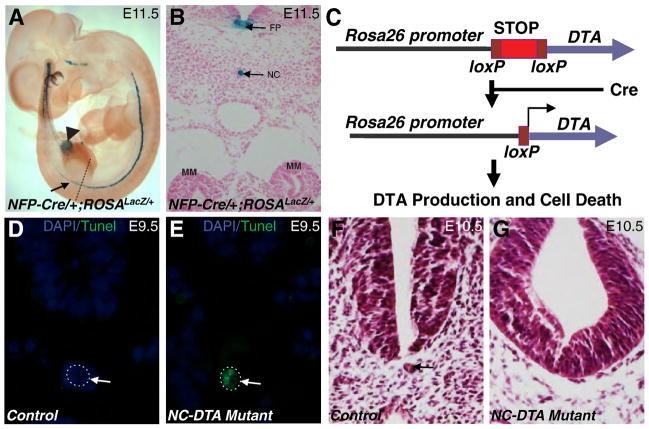
Fig. 1. Transgenic ablation of the notochord and floor plate by diphtheria toxin (DTA).
A. The ROSALacZ reporter revealed the expression of NFP-Cre in notochord and floor plate (arrow). The weak expression was also observed in part of the hindgut (arrowhead). B, A transverse section at the level of hind limb bud (shown by dashed line in Fig. 1A) showed specific X Gal staining in the notochord and floor plate. C, when combined with a ROSADTA allele, NFP-Cre induces the production of DTA, translational arrests, and apoptosis in Cre-positive cells. TUNEL assay on transverse sections of E9.5 control (D) and mutant (E) embryos at the level of the MM. Notochords are outlined by dotted circles. Apoptotic cells were seen only in the notochord of the mutant (E). F and G, transverse sections of the control (F) and mutant (G) embryos revealed notochord degeneration in the mutants at E10.5. Arrow in D–F points to the notochord. FP: floor plate; NC: notochord.
Histological Analysis, Apoptosis Assay, and Skeletal Preparations
For histological analyses, embryos were either fixed with 4% paraformaldehyde and embedded in paraffin or were cryopreserved in OCT. 7 μm paraffin sections or 10 μm cryosections were collected and stained by H&E following standard protocol (McDill et al., 2006). β-galactocidase assays on whole-mount preparations and sections were performed as described (Wang et al., 2009). Terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling (TUNEL) analysis was performed on paraffin-embedded sections by using the ApopTag plus peroxidase in situ apoptosis detection kit (Roche, NutleY, NJ) (Jia et al., 2008). Skeletal preparations of newborn pups were made as described (Chen and Capecchi, 1997). Briefly, after removal of skin, newborn mice were eviscerated and fixed in 100% ethanol. The samples were then stained with 0.03% Alcian Blue and 0.03% Alizarin Red. After staining, the samples were treated with 2% potassium hydroxide and cleared in a series of solutions with increasing concentration of glycerol.
Immunohistochemistry
Paraffin sections were stained with a rabbit polyclonal anti-Pax2 antibody (Covance PRB-276P, 1:200), and a mouse monoclonal anti-αSMA antibody (Sigma A2547, 1:200). Appropriate AlexaFlour488 or 555-conjugated secondary antibodies (Molecular Probe, 1:1000) were used to detect the corresponding primary antibodies. Whole-mount immunostaining was carried out with the anti-Pax2 antibody as described (Chang et al., 2004). To analyze vessel patterning, India ink was injected into the left ventricle with glass needles and cleared in 1:2 benzyl alcohol/benzyl benzoate (Jia et al., 2007). Alcian blue staining of the paraffin sections was done by incubating the dewaxed sections with 1% Alcian blue staining solution for 15 minutes at room temperature. For the metric data of MM positions, cross sections near the hind limb level were prepared from control and mutant embryos. These sections were stained by an anti-Pax2 antibody to reveal the nephric structures. The closest distance between left and right MM tissues was measured using an image of a scale taken under the same magnification as the embryo cross sections.
RNA In situ hybridization
Whole-mount RNA in situ hybridization was performed as previously described (Jia et al., 2007). 3′ UTR regions of the genes Pax2, Gdnf, Foxd1, Shh, Sox9 and Id2 were amplified by PCR with the T7 promoter attached to the 5′ primers. The amplicons were used as templates for making the RNA probes using the MAXIscript in vitro transcription kit (Ambion, Austin, TX). RNA in situ hybridization was performed on 10 μm cryostat sections.
Results
Genetically controlled cell ablation in the notochord and floor plate
To examine the potential functional role of the axial structures, especially the notochord, on kidney development, we disrupted the notochord and floor plate by using a NFP (notochord and floor plate)-Cre transgene (Kumar et al., 2007) to direct DTA-mediated apoptosis in these structures (Fig. 1). As revealed by the ROSALacZ reporter (Soriano, 1999), the expression of NFP-Cre can be detected in the notochord and floor plate (Fig. 1A). Part of the hindgut was also positive for Cre (Fig. 1A, arrowhead). However, Cre expression in the hindgut at the MM level was undetected (Fig. 1A). NFP-Cre has no detectable expression in the kidney, ureter, or their progenitors during embryogenesis (Fig. 1A–B, and data not shown). We combined the NFP-Cre transgene and a ROSADTA allele (Wu et al., 2006) to produce NFP-Cre/+;ROSADTA/+ and later NFP-Cre/+;ROSADTA/DTA mice. Since NFP-Cre/+;ROSADTA/+ and NFP-Cre/+;ROSADTA/DTA mice have similar phenotypes, we refer both as the NC-DTA mutants for simplicity. In Cre-positive cells, Cre-mediated loxP recombination removes a transcriptional “STOP” cassette in the ROSADTA allele, leading to the expression of DTA under the control of the ROSA promoter (Fig. 1C) (Jia et al., 2008; Wu et al., 2006). DTA can cause translational arrest through its inhibition of the eukaryotic elongation factor 2. Affected cells may lose some of their functions, including the ability to signal to adjacent cells, long before their final demise (Jia et al., 2008; Wu et al., 2006). As rodent cells have no DTA receptors, even if DTA escapes from a dying cell, its neighboring cells are not affected (Wu et al., 2006). Cre positive cells were reportedly detected in the notochord as patches at as early as E7.5 (Kumar et al., 2007). The expression of Cre in the notochord becomes continuous by E9.5 (Kumar et al., 2007). TUNEL assay showed apoptotic cells in the notochord by E9.5 in NC-DTA mutant embryos (Fig. 1D–E). The floor plate and notochord were severely disrupted or absent in these mutant embryos from E10.5 onward (Fig. 1F–G). The neural tube appeared to be more rounded in the mutants compared to the controls (Fig. 1F–G), apparently as a result of the lack of the floor plate. The hindgut, however, showed no detectable differences between the controls and mutants, possibly because Cre expression in the hindgut is restricted and weak (Fig. 1A–B and data not shown).
Disruption of notochord and floor plate causes axial defects and kidney fusion but not renal agenesis
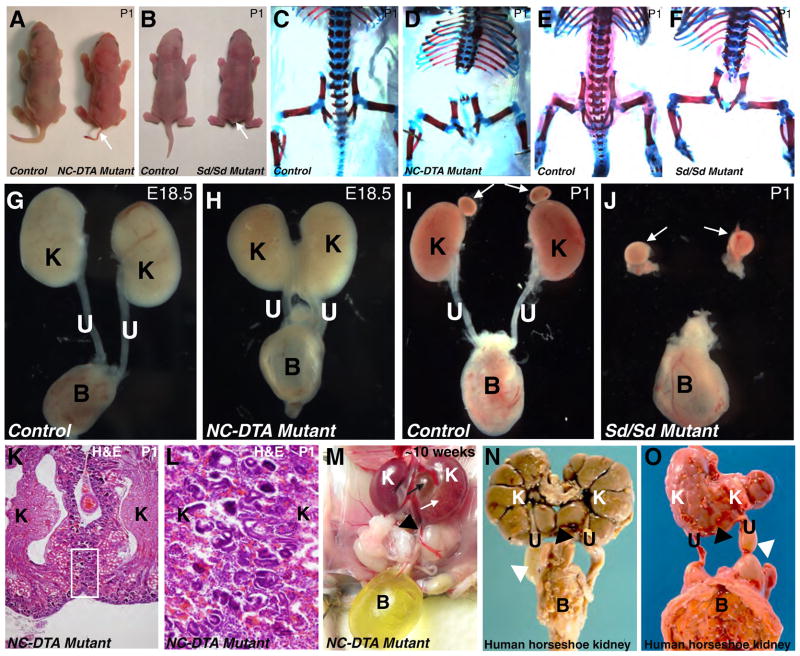
79% (15/19) NC-DTA mutants have a short tail with little or no skeletal support (Fig. 2A), bearing marked resemblance to defects in Danforth short tail homozygous (Sd/Sd) mutants (Fig. 2B) (Danforth, 1930). The vertebral column below the thoracic level is deformed or essentially missing in these NC-DTA mutants (Fig. 1C–D). These abnormalities bear marked resemblance to defects in Sd/Sd mutants (Fig. 1E–F). However, to our surprise and unlike the renal agenesis in Sd/Sd mutants, the NC-DTA mutants have kidneys (Fig. 2G–J). Interestingly, about 80% (28/35) of the NC-DTA mutants have fused kidneys resembling horseshoe kidneys found in human patients (Segura et al., 1972). The fusion of the left and right kidneys is not limited to the joining of the renal capsules. Renal cortices from both kidneys were connected as revealed by histological analysis (Fig. 2K–L). When adult mice were analyzed, 25% (1/4) of the fused kidneys exhibit hydronephrosis and hydroureter (Fig. 2M) that are also observed in human patients with horseshoe kidneys (Fig. 1N–O). In addition to problems associated with the defective vertebral column, hydronephrosis and hydroureter may also account for higher mortality in the NC-DTA mutants as these defects can lead to obstructive nephropathy, renal failure, and death (Chen, 2009; Chevalier, 1999).
Fig. 2. DTA mediated notochord and floor plate ablation results in kidney fusion but not renal agenesis.
A, Newborn control and NC-DTA mutant mice. B, Newborn wild-type and Danforth Short tail homozygous (Sd/Sd) mutants. Both NC-DTA mutants and Sd/Sd mutants have short tails (arrows in A and B). C–F, Skeletal preparations from newborn control (C) and NC-DTA mutant mice (D). In NC-DTA mutants, vertebral column is severely defective below the thoracic level, similar to skeletal defects in Sd/Sd mutants (E–F). Urinary systems from control mice (G and I), a NC-DTA mutant (H) and a Sd/Sd mutant (J). The NC-DTA mutants have fused kidneys resembling horseshoe kidneys (H), different from Sd/Sd mutants that have bilateral renal agenesis (J). White arrows indicate adrenal glands. K, H&E stained paraffin section of the fused kidneys. The area within the rectangle is shown in L at a higher magnification. Cortices from left and right kidneys are connected. M, a NC-DTA mutant with fused and hydronephrotic kidney. White arrow points to hydronephrotic kidney. Black arrow points to expanded pelvic area. N–O, Horseshoe kidneys in human fetus. Black triangle points to the fused lower poles of the left and right kidneys and white triangle points to hydroureter. K: kidney, U: ureter, B: bladder.
Mediolateral position of the MM is affected by axial disruption
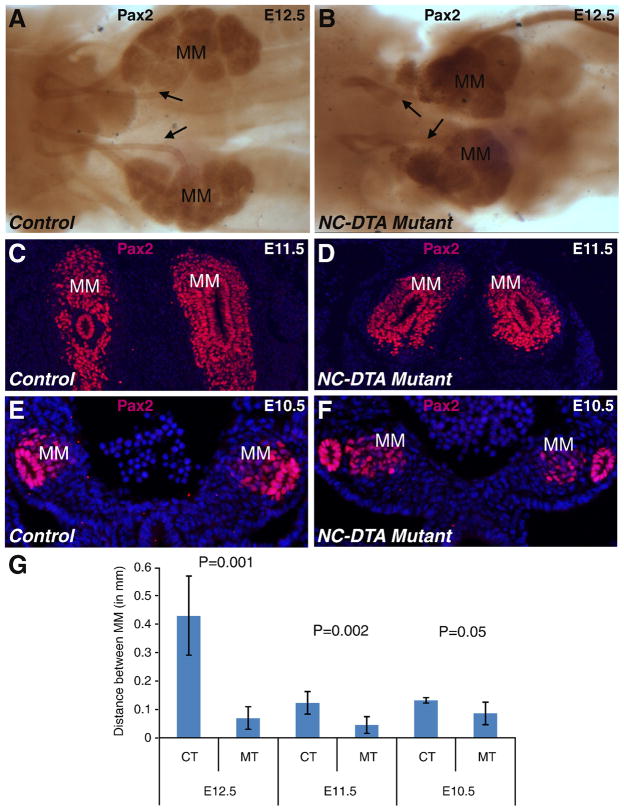
To investigate the developmental basis for the malformed kidneys in the NC-DTA mutants, we examined metanephric kidney development during midgestation, a period critical for the patterning of this organ. Dissected urogenital ridge immunostained for Pax2 showed that the mutant MM cell masses from both sides are positioned much closer to the midline (and to each other), than those in the control at E12.5 (Fig. 3). This finding indicates that disruption of the notochord and floor plate affects the mediolateral positioning of the MM, setting the stage for kidney fusion to occur. The position of the MM along the anterior-posterior axis remains at the same level near the hind limb bud in the mutants. The more medial positioning of the MM was also detected by Pax2 antibody staining on transverse sections at E11.5 (Fig. 3C–D) and even at E10.5, when the MM first appears (Fig. 3E–F). Metric data also showed that the distance between the left and right MM tissues at early developmental stages was significantly shorter in the mutants than in the controls (Fig. 3G).
Fig. 3. Disruption of the notochord and floor plate affects the mediolateral positioning of the MM.
A–B, Pax2 immunostaining outlined the MM, WD, and UB at E12.5 in the control sample (A) and the mutant sample (B). C–F, Anti-Pax2 antibody stained transverse sections of E11.5 control (C) and mutant (D) and E10.5 control (E) and mutant (F) embryos. DAPI staining highlights the nuclei. G, Metric data measuring the distance between MM tissues from both sides. The MM tissues are closer to each other in the mutants (two-tailed t-test). Distance between the left and right MM cell masses (in millimeters) at E12.5: CT 0.431667±0.14 (n=4), MT 0.07±0.04 (n=4), P=0.001; at E11.5: CT 0.123333±0.04 (n=4), MT 0.045±0.03 (n=4), P=0.002; at E10.5: CT 0.132±0.01 (n=4), MT 0.086±0.04 (n=5), P=0.05. MM: metanephric mesenchyme; CT: control; MT: mutant. Arrow: UB/ureter.
Altered differentiation of the perinotochordal mesoderm and the repatterning of the dorsal aorta in embryos with axial disruption
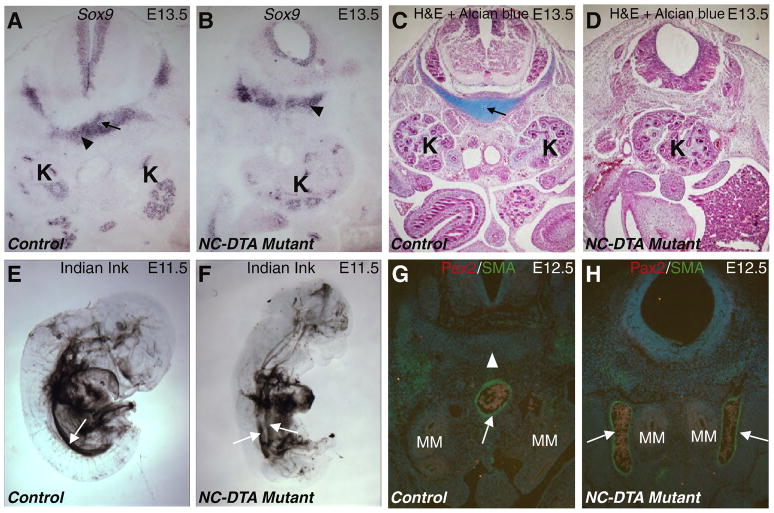
To investigate if the disruption of the notochord leads to a general absence of the perinotochordal mesoderm, we used Sox9 to label this population of cells. Sox9-positive perinotochordal cells did not show a drastic change in size and position in the mutants at E13.5 (Fig. 4A–B). However, while the perinotochordal cells soon became Alcian blue positive, the mutant cells in the corresponding region remained negative for Alcian blue (Fig. 4C–D). These results indicate that the perinotochordal mesoderm persisted in the absence of the notochord but failed to undergo chondrogenesis. The dorsal aortae running in parallel and lateral to the notochord became a single midline descending aorta throughout the posterior half of the embryos by E11.5 (Jia et al., 2007), as revealed by Indian ink injection labeling the major vessels in the control (Fig. 4E). However, the dorsal aortae remained separated in the posterior part of the NC-DTA mutant embryos (Fig. 4F) at this time. On transverse paraffin sections, there was a single midline descending aorta in the control at the level of the MM at E12.5 (Fig. 4G). While the MM domains from both sides were positioned closer to the midline (and each other), the dorsal aortae remained well separated in about 73% (8/11) of the mutants (Fig. 4H). The presence of Sox9-expressing cells (Fig. 4B), the separation of the dorsal aortae (Fig. 4F, H), and the separation of the WDs (Fig. 3) and other bilateral structures (such as adrenal glands, testis, seminal vesicles, ovaries, etc) suggest that the alterations in the midline structures due to notochord and floor plate ablation do not always cause fusion or closer proximity between other bilateral structures. The elimination of axial sources of regulators (such as Shh, Noggin, etc) appears to alter the developmental program for midline structures, which in turn, affect the mediolateral positioning of not just the MM, but also the dorsal aortae. Although a possible link between aortae patterning and metanephric kidneys positioning exists, the observation that 27% (3/11) of the mutants with fused kidneys had single dorsal aorta argues against a simple causal relation between failure in aortae merger and the fusion of the kidneys.
Fig. 4. Disruption of the notochord and floor plate affects chondrogenesis of perinotochordal cells and midline vascular development.
A–B, Sox9 RNA in situ hybridization on transverse sections at the MM level in control (A) and mutant (B) E13.5 embryos. Black arrow in A points to the notochord that is absence in the mutant (B). Black triangles point to the perinotochordal cells positive for Sox9 in both control and mutant samples. C–D, Transverse sections at the MM level in control (C) and mutant (D) E13.5 embryos stained with H&E and Alcian blue (for cartilage). Black arrow in C points to the notochord that is absence in the mutant (D). E–F, India ink injection into the left ventricle of E11.5 control (E) and mutant (F) embryos. The white arrows point to the dorsal aorta. At this time, the control dorsal aorta does not have major branches below the aortic arches (E). However, the mutant aorta is still bifurcated for most of the trunk region (F). G–H, transverse sections at the MM level from control (G) and NC-DTA mutant (H) E12.5 embryos. White arrows point to the αSMA staining (Green) in the aortic wall. White triangle points to the notochord (absent in the mutant). MM is highlighted by Pax2 staining in red. MM: metanephric mesenchyme; K: kidney.
Notochord and floor plate regulate signaling responses of the midline cells but not nephrogenesis within the kidney proper
To further determine the molecular changes that may be involved in the formation of the fused kidneys, we examined the expression of genes important for nephrogenesis and the development of the paraxial structures. We found that the expression of Pax2 and Gdnf was not significantly changed within the kidney proper in the mutants (Fig. 5A–D). Foxd1 is important for the formation of the renal capsule and is also indispensable for nephrogenesis, Mice deficient for Foxd1 have arrested development of the nephrons and fusion of the kidneys through the joining of the renal capsules (Levinson et al., 2005). Foxd1 shows a medial expansion of its expression domain in mutants (Fig. 5E–F). The medial expansion of the Foxd1 expression domain goes beyond the Pax2-positive MM, as revealed by Pax2 RNA in situ hybridization on nearby sections (Fig. 5A–B). This reflects the Foxd1 expression outside the MM in the progenitor cells of the renal capsule and in other mesodermal cells. Except for the mediolateral position, the expression of Foxd1 in the developing MM is not significantly different between the control and the mutants. This is consistent with the finding of no arrest of nephrogenesis in the NC-DTA mutants.
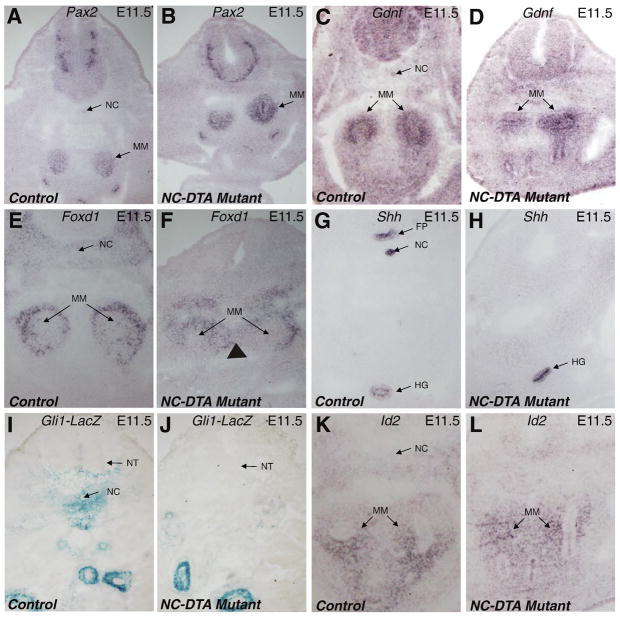
Fig. 5. Axial disruption affects the mediolateral positioning of the MM, but has little impact on the nephrogenic program inside the kidneys.
Pax2 RNA in situ hybridization outlines the MM from the control (A) and mutant (B) at E11.5. C–D, expression patterns of Gdnf in MM are similar between control and mutant at E11.5. Foxd1 expression in the outer portion of the MM and other mesodermal cells, including cells that contribute to the formation of the renal capsule, is apparent in both the control (E) and the mutant (F) samples. Arrowhead marks a medial expansion of its expression domain in mutant. Shh is normally expressed in the floor plate, notochord, and the hindgut (G). In the mutant, the expression in the floor plate and notochord is missing but the expression in the hindgut remains (H). Expression of Gli1lacZ is seen around notochord in the control (I) but there is a drastic reduction of Gli1lacZ positive cells in the midline cell populations in the mutant sample. (J). The expression pattern of Id2 is similar in control and mutants (K–L). FP: floor plate; HG: hindgut; MM: metanephric mesenchyme; NC: notochord; NT: Neural tube.
Shh is specifically expressed in the notochord and floor plate (Fig. 5G). Disruption of these structures essentially eliminated the axial source of Shh (Fig. 5H). However, the expression of Shh in the hindgut is not significantly changed, consistent with the lack of detectable hindgut defects in the NC-DTA mutants. Gli1 expression can reflect cellular response to Shh signaling (Bai et al., 2002). Expression of a Gli1lacZ allele (Bai et al., 2002) is observed in tissues around the notochord in controls (Fig. 5I), but absent in mutants (Fig. 5J), indicating a much reduced Shh influence in these cell populations potentially important for the separation of the left and right MM cell masses. The Bmp inhibitor Noggin has specific expression in the notochord. This expression was also eliminated when the notochord and floor plate were disrupted (data not shown). Since Noggin inhibits Bmp signaling, we examined the possibility that the absence of the notochord (a specific source of Noggin) would lead to an increase of Bmp signaling in nearby tissues. The expression of Id genes is tightly regulated by Bmps and can be used as a readout for Bmp signaling (Jia et al., 2007). In addition, Id2 has been suggested to play a role in kidney development (Aoki et al., 2004). We thus examine the expression of Id2 in NC-DTA mutants. We found that the expression pattern of Id2 was not drastically changed in the perinotochordal area in these mutants (Fig. 5K–L), suggesting that the direct effect of axial ablation on Bmp signaling in nearby cells is more limited than those on Hedgehog signaling.
Inactivation of Shh in axial structure causes kidney fusions similar to those of the NC-DTA mutants
To further investigate if axial Shh sources affect metanephric kidney development, we specifically deleted Shh in the notochord and floor plate by combining the NFP-Cre transgene with a floxed-Shh allele (Fig. 6A–B) (Yu et al., 2002). We did not detect any abnormalities in mice with heterozygous deletion of Shh in the notochord and floor plates. However, in 90% (9/10) of the mice with homozygous deletion of Shh in these structures (NFP-Cre/+; ShhloxP/loxP, referred to as NC-Shh for simplicity), we observed MM positional abnormalities and kidney fusions similar to those seen in the NC-DTA mutants described above (Fig. 6D). Fusion of the left and right MM cell masses is apparent at E12.5, even in the presence of the notochord (Fig. 6C–F). The effects of the axial deletion of Shh on MM positioning became apparent at E11.5 (Fig. 6). The Pax2-positive MM tissues from both sides were positioned more medially at this time (Fig. 6). Ptch1 encodes the receptor for Shh and is upregulated in cells responding to Shh signals. Ptch1 expression was reduced around the notochord in mutants lacking axial Shh (Fig. 6I–J), indicating a much reduced Shh influence in these cell populations potentially important for the separation of the left and right MM cell masses. These results further support that Shh from the notochord and floor plate may provide the key signal for the development of the midline structures important for positioning the MM.
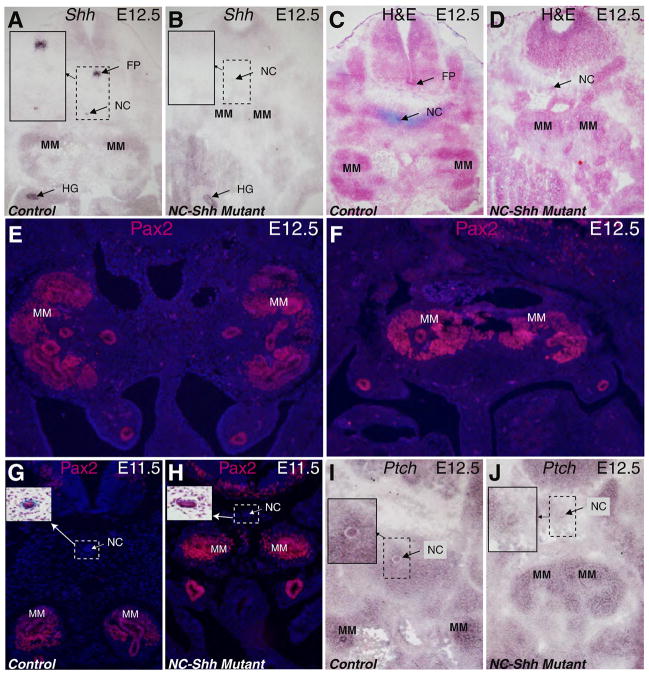
Fig. 6. Inactivation of Shh in the notochord and floor plate also leads to kidney fusion but not renal agenesis.
Shh expression is detected in the notochord, floor plate and the hindgut on the transverse section at the MM level in the E12.5 control embryo (A). Shh expression in the notochord and floor plate is absent in the littermate mutant (B), though the notochord is still present at this stage. Insets show the enlarged images of the rectangular areas. H&E and Alcian blue (for cartilage) stained transverse sections from control (C) and mutant (D) embryos at E12.5 showed that the notochord persists in NC-Shh mutants with the MM tissues fused. Pax2 staining on E12.5 cross sections shows the separation of the left and right MM tissues in the control (E) and the fused MM tissues in the mutant (F). Pax2 staining on E11.5 control (G) and mutant (H) littermates revealed that MM tissues are closer to each other in the mutants. Insets show notochord images from H&E stained adjacent sections. Compared to the control (I) at E12.5, Ptch1 expression in the mutant sample (J) is drastically decreased in perinotochordal mesoderm at the MM level (Insets show the enlarged images of the rectangular areas.). FP: floor plate; HG: hindgut; MM: metanephric mesenchyme; NC: notochord.
Discussion
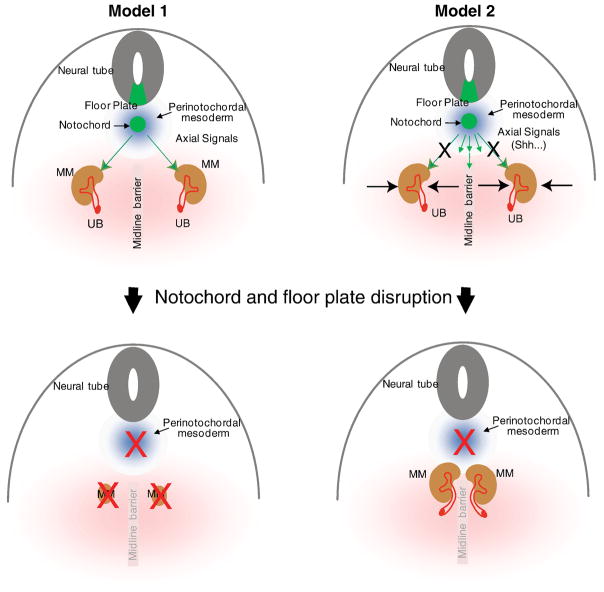
Study of the Sd mutants with concurrent notochord defect and renal agenesis suggested that the disruption of the notochord may abort nephrogenesis (Maatman et al., 1997) (Fig. 7, Model 1). Surprisingly, our study has shown that ablation of the notochord/floor plate and specific inactivation of Shh in these structures causes kidney fusion but not agenesis (Fig. 2–6). As depicted in Figure 7, Model 2, our results suggest that axial signals have no direct impact on nephrogenesis. Instead, loss of the axial signals, due to cell ablation or gene inactivation, results in signaling interruptions and developmental alterations in midline cell populations. Such alterations disrupt the organization and differentiation of structures along the mediolateral axis, resulting in more medially positioned MM cell masses and the eventual fusion of left and right kidneys. A conceptual “midline barrier” during normal development may help to prevent fusion of the kidneys. Such a barrier may consist of midline mesoderm forming a physical separation and/or repulsive signals originated from these midline cells. Thus, in a way, the effects of midline signals from axial structures, especially the notochord and the floor plate, on metanephric kidney development appear to be maintaining an effective “midline barrier” and helping to determine the final mediolateral position of the kidneys.
Fig. 7. Models for axial regulation of metanephric kidney development.
The finding of renal fusion in the NC-DTA mutants contradicts the hypothesis that degeneration of the notochord leads to renal agenesis (Model 1). In a new model (Model 2), signals from the notochord and floor plate, such as Shh, do not directly affect nephrogenesis. Instead, these signals affect the development of the midline structures and the positioning of the MM. See text for details. UB: ureteric bud; MM: metanephric mesenchyme.
Foxd1 has been shown to have an important role in the formation of the renal capsule and stroma (Hatini et al., 1996; Levinson et al., 2005). Mice lacking Foxd1 develop kidney fusion largely due to a failure of the developing kidney to separate from the body wall (Levinson et al., 2005). A recent study showed that the paraxial mesoderm contributed Foxd1-positive stromal cells to the developing kidney and surgical ablation of paraxial mesoderm led to fused metanephroi in over half of the surviving embryos (Guillaume et al., 2009). In the NC-DTA mutants, the expression of Foxd1 within the MM was not altered and its medially shifted expression domain appears to be simply due to overall change in MM position. A clear difference between the NC-DTA mutants and the Foxd1 mutants is the nephrogenesis within the kidney proper. Severe defects in branching morphogenesis and nephron differentiation were reported in the Foxd1 mutants (Hatini et al., 1996; Levinson et al., 2005). Conversely, nephrogenesis in the NC-DTA mutants is largely unaffected as all nephron elements are present and the fused kidneys are apparently functional unless hydronephrosis develops later in life (Figs. 2). In addition, the kidneys of the Foxd1 mutants fail to ascend, leaving the kidneys in a pelvic position far away from the adrenal glands. The kidneys of the NC-DTA mutants, however, ascend properly to the high lumbar position immediately caudal to the adrenal glands. Apparent kidney fusion was reported in mice homozygous for a Fgfr1 (Fibroblast growth factor receptor 1) mutation (Fgfr1ΔFrs) (Hoch and Soriano, 2006). Interestingly, a portion of the Fgfr1ΔFrs/ΔFrs mutants had caudal truncation with notochord involvement up to the hind limb bud level where MM forms. It is unclear if notochord truncation and kidney fusion were tightly linked in these mutants. If it is, then the kidney fusion may be a result of notochord defect, similar to the NC-DTA mutants described here. Alternatively, caudal truncation may also create spatial constraints leading to kidney fusion without notochord involvement. Taken together, it appears that kidney fusion can result from disparate molecular alteration and perturbations in different structures.
In the ablation model (NC-DTA mice), the notochord is completely absent at the level of the MM at E10.5, a situation very similar to, or slightly more severe than that observed in the Danforth’s short tail (Sd/Sd) mutants (Danforth, 1930) with kidney agenesis. Thus, the finding of kidney fusion in the NC-DTA mice indicates that the notochord defect in Sd/Sd mutants is not the major cause for renal agenesis. Instead, the Sd mutation may have a more direct role in the nephric progenitors for kidney development. The role of the hindgut in the development of the metanephric kidney was not directly addressed in the current study. The NFP-Cre transgene used did not induce significant changes in the hindgut to allow an assessment of its role in the patterning of the MM and UB for kidney development. This, however, provides a clear separation of effects from the notochord/floor plate and the hindgut.
Germline inactivation of Shh results in severe developmental anomalies throughout the embryo (Chiang et al., 1996). In the urinary system, both aplasia and pelvic kidney (kidney located in the pelvic region) were reported in the Shh−/− embryos (Hu et al., 2006). Such severe anomalies in kidney development are conceivably the combined results from the loss of Shh function in kidney and non-renal structures. Shh has been found to be important in the UB epithelium for the development of the ureter and kidney (Yu et al., 2002). However, Shh expression in the UB is unlikely to play a key role in determining the position of the MM, as suggested by the normal positioning of the kidneys in mice with UB-specific inactivation of Shh (Yu et al., 2002). The drastic decrease of Shh response in midline cell populations in NC-DTA mutants and similar kidney fusion defects found in embryos with notochord and floor plate-specific inactivation of Shh (NC-Shh) suggest that Shh from these axial structures has major influence on the growth and differentiation of the midline cell population and the positioning of the kidney. While the notochord is completely absent in the NC-DTA mutants, it is still present at E11.5 and E12.5 in the NC-Shh mutants at the level of the MM, suggesting that Shh is the key factor emanating from the notochord and floor plate for their influence on the position of the kidney. In addition to being an important source of Shh, the notochord also secretes inhibitors for Bmp signaling, such as Noggin and Chordin. However, Id2 expression, as a read-out for responses to Bmp signaling in the perinotochordal mesoderm and other midline cell populations did not show drastic changes in the mutants (Fig. 5). The absence of changes in Bmp response when Noggin and Chordin are eliminated from the notochord is likely due to the presence of other Bmp inhibitors in the area, such as Gremlin.
In conclusion, this study demonstrates that the notochord and floor plate are dispensable for nephrogenesis but required for the correct positioning of the metanephric kidney. Disruption of the notochord and floor plate or Shh from these structures can affect mediolateral organization of developing midline and lateral structures and lead to a more medial position for the MM, setting the stage for kidney fusions to occur. The fused kidneys resemble the horseshoe kidneys seen in human patients with congenital anomaly of the kidney and urinary tract (CAKUT) (Miyazaki and Ichikawa, 2003). Although horseshoe kidneys do not by themselves affect renal filtration functions, they are prone to develop hydronephrosis and hydroureter that can lead to renal failure and mortality. This study provides insights into how certain congenital kidney defects can be caused by anomalies outside the renal primordial and raises the possibility that mediolateral patterning defect during embryogenesis can be a potential cause for horseshoe kidneys in human patients.
Acknowledgments
We thank Dr. Stephen Bonsib (Louisiana State University Health Sciences Center) for providing the images of the horseshoe kidneys in humans. F.C. is supported in part by institutional funds from the Department of Internal Medicine/Renal Division at Washington University School of Medicine and NIH grants (DK81592 and DK67386). We also thank the Georg M. O’Brien Center for Kidney Disease Research at Washington University (P30DK079333) for core services.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aoki Y, Mori S, Kitajima K, Yokoyama O, Kanamaru H, Okada K, Yokota Y. Id2 haploinsufficiency in mice leads to congenital hydronephrosis resembling that in humans. Genes Cells. 2004;9:1287–96. doi: 10.1111/j.1365-2443.2004.00805.x. [DOI] [PubMed] [Google Scholar]
- Bai CB, Auerbach W, Lee JS, Stephen D, Joyner AL. Gli2, but not Gli1, is required for initial Shh signaling and ectopic activation of the Shh pathway. Development. 2002;129:4753–61. doi: 10.1242/dev.129.20.4753. [DOI] [PubMed] [Google Scholar]
- Barak H, Rosenfelder L, Schultheiss TM, Reshef R. Cell fate specification along the anterior-posterior axis of the intermediate mesoderm. Dev Dyn. 2005;232:901–14. doi: 10.1002/dvdy.20263. [DOI] [PubMed] [Google Scholar]
- Chang CP, McDill BW, Neilson JR, Joist HE, Epstein JA, Crabtree GR, Chen F. Calcineurin is required in urinary tract mesenchyme for the development of the pyeloureteral peristaltic machinery. J Clin Invest. 2004;113:1051–8. doi: 10.1172/JCI20049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F. Genetic and developmental basis for urinary tract obstruction. Pediatr Nephrol. 2009;24:1621–32. doi: 10.1007/s00467-008-1072-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F, Capecchi MR. Targeted mutations in hoxa-9 and hoxb-9 reveal synergistic interactions. Dev Biol. 1997;181:186–96. doi: 10.1006/dbio.1996.8440. [DOI] [PubMed] [Google Scholar]
- Chevalier RL. Molecular and cellular pathophysiology of obstructive nephropathy. Pediatr Nephrol. 1999;13:612–9. doi: 10.1007/s004670050756. [DOI] [PubMed] [Google Scholar]
- Chiang C, Litingtung Y, Lee E, Young KE, Corden JL, Westphal H, Beachy PA. Cyclopia and defective axial patterning in mice lacking Sonic hedgehog gene function. Nature. 1996;383:407–13. doi: 10.1038/383407a0. [DOI] [PubMed] [Google Scholar]
- Danforth CH. Developmental anamolies in a special strain of mice. Amer Jour Anat. 1930;45:275–287. [Google Scholar]
- Dressler GR. The cellular basis of kidney development. Annu Rev Cell Dev Biol. 2006;22:509–29. doi: 10.1146/annurev.cellbio.22.010305.104340. [DOI] [PubMed] [Google Scholar]
- Guillaume R, Bressan M, Herzlinger D. Paraxial mesoderm contributes stromal cells to the developing kidney. Dev Biol. 2009;329:169–75. doi: 10.1016/j.ydbio.2009.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatini V, Huh SO, Herzlinger D, Soares VC, Lai E. Essential role of stromal mesenchyme in kidney morphogenesis revealed by targeted disruption of Winged Helix transcription factor BF-2. Genes Dev. 1996;10:1467–78. doi: 10.1101/gad.10.12.1467. [DOI] [PubMed] [Google Scholar]
- Hoch RV, Soriano P. Context-specific requirements for Fgfr1 signaling through Frs2 and Frs3 during mouse development. Development. 2006;133:663–73. doi: 10.1242/dev.02242. [DOI] [PubMed] [Google Scholar]
- Hu MC, Mo R, Bhella S, Wilson CW, Chuang PT, Hui CC, Rosenblum ND. GLI3-dependent transcriptional repression of Gli1, Gli2 and kidney patterning genes disrupts renal morphogenesis. Development. 2006;133:569–78. doi: 10.1242/dev.02220. [DOI] [PubMed] [Google Scholar]
- James RG, Schultheiss TM. Patterning of the avian intermediate mesoderm by lateral plate and axial tissues. Dev Biol. 2003;253:109–24. doi: 10.1006/dbio.2002.0863. [DOI] [PubMed] [Google Scholar]
- Jia Q, McDill BW, Li SZ, Deng C, Chang CP, Chen F. Smad signaling in the neural crest regulates cardiac outflow tract remodeling through cell autonomous and non-cell autonomous effects. Dev Biol. 2007;311:172–84. doi: 10.1016/j.ydbio.2007.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia Q, McDill BW, Sankarapandian B, Wu S, Liapis H, Holzman LB, Capecchi MR, Miner JH, Chen F. Ablation of developing podocytes disrupts cellular interactions and nephrogenesis both inside and outside the glomerulus. Am J Physiol Renal Physiol. 2008;295:F1790–8. doi: 10.1152/ajprenal.90519.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Yamaguchi T, Sharma P, Kuehn MR. Transgenic mouse lines expressing Cre recombinase specifically in posterior notochord and notochord. Genesis. 2007;45:729–36. doi: 10.1002/dvg.20346. [DOI] [PubMed] [Google Scholar]
- Levinson RS, Batourina E, Choi C, Vorontchikhina M, Kitajewski J, Mendelsohn CL. Foxd1-dependent signals control cellularity in the renal capsule, a structure required for normal renal development. Development. 2005;132:529–39. doi: 10.1242/dev.01604. [DOI] [PubMed] [Google Scholar]
- Maatman R, Zachgo J, Gossler A. The Danforth’s short tail mutation acts cell autonomously in notochord cells and ventral hindgut endoderm. Development. 1997;124:4019–28. doi: 10.1242/dev.124.20.4019. [DOI] [PubMed] [Google Scholar]
- Mauch TJ, Yang G, Wright M, Smith D, Schoenwolf GC. Signals from trunk paraxial mesoderm induce pronephros formation in chick intermediate mesoderm. Dev Biol. 2000;220:62–75. doi: 10.1006/dbio.2000.9623. [DOI] [PubMed] [Google Scholar]
- McDill BW, Li SZ, Kovach PA, Ding L, Chen F. Congenital progressive hydronephrosis (cph) is caused by an S256L mutation in aquaporin-2 that affects its phosphorylation and apical membrane accumulation. Proc Natl Acad Sci U S A. 2006;103:6952–7. doi: 10.1073/pnas.0602087103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki Y, Ichikawa I. Ontogeny of congenital anomalies of the kidney and urinary tract, CAKUT. Pediatr Int. 2003;45:598–604. doi: 10.1046/j.1442-200x.2003.01777.x. [DOI] [PubMed] [Google Scholar]
- Placzek M. The role of the notochord and floor plate in inductive interactions. Curr Opin Genet Dev. 1995;5:499–506. doi: 10.1016/0959-437x(95)90055-l. [DOI] [PubMed] [Google Scholar]
- Schedl A. Renal abnormalities and their developmental origin. Nature Reviews genetics. 2007;8:791–802. doi: 10.1038/nrg2205. [DOI] [PubMed] [Google Scholar]
- Segura JW, Kelalis PP, Burke EC. Horseshoe kidney in children. J Urol. 1972;108:333–6. doi: 10.1016/s0022-5347(17)60732-8. [DOI] [PubMed] [Google Scholar]
- Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nature Genetics. 1999;21:70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- Stemple DL. Structure and function of the notochord: an essential organ for chordate development. Development. 2005;132:2503–12. doi: 10.1242/dev.01812. [DOI] [PubMed] [Google Scholar]
- Wang Y, Tripathi P, Guo Q, Coussens M, Ma L, Chen F. Cre/lox recombination in the lower urinary tract. Genesis. 2009;47:409–413. doi: 10.1002/dvg.20515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S, Wu Y, Capecchi MR. Motoneurons and oligodendrocytes are sequentially generated from neural stem cells but do not appear to share common lineage-restricted progenitors in vivo. Development. 2006;133:581–90. doi: 10.1242/dev.02236. [DOI] [PubMed] [Google Scholar]
- Yu J, Carroll TJ, McMahon AP. Sonic hedgehog regulates proliferation and differentiation of mesenchymal cells in the mouse metanephric kidney. Development. 2002;129:5301–12. doi: 10.1242/dev.129.22.5301. [DOI] [PubMed] [Google Scholar]