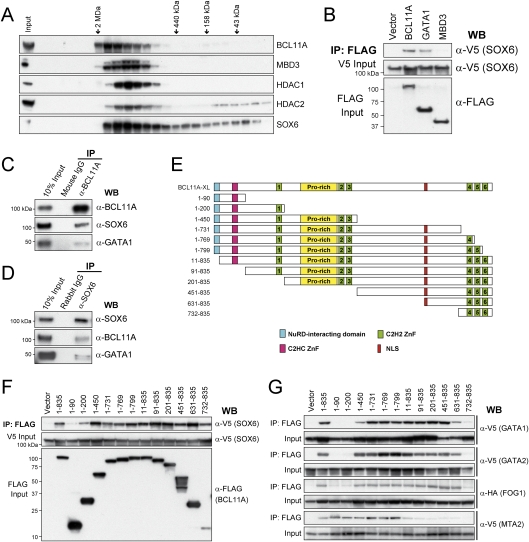
Figure 4.
Physical interaction between BCL11A and SOX6. (A) Gel filtration fractions of nuclear extracts from human erythroid progenitors (day 5 in differentiation medium) were blotted for BCL11A, MBD3, HDAC1, HDAC2, and SOX6. There is significant overlap between BCL11A, SOX6, and components of the Mi-2/NuRD complexes. Elution positions of molecular mass standards (from left to right, 2 MDa, 440 kDa, 158 kDa, and 43 kDa) are indicated. (B) V5-tagged SOX6 cDNA was coexpressed in COS7 cells with a Flag-tagged empty vector, BCL11A, GATA1, and MBD3 cDNA. Nuclear extracts were immunoprecipitated using anti-Flag antibody, and copurified proteins were analyzed by Western blot with anti-V5 antibody. Inputs (5%) are shown. (C) Endogenous SOX6 and GATA1 were coimmunoprecipitated with BCL11A in human erythroid cells. Nuclear extracts from erythroid progenitors (day 5 in differentiation medium) were immunoprecipitated with anti-BCL11A (14B5) antibody or mouse IgG (negative control), and copurified proteins were analyzed by Western blot with antibodies against BCL11A, SOX6, and GATA1. (D) Endogenous BCL11A was coimmunoprecipitated with SOX6 in human erythroid cells. Nuclear extracts from human erythroid progenitors were immunoprecipitated with anti-SOX6 antibody or rabbit IgG (negative control). (E) Schematic diagram of BCL11A constructs used in F and G. (F) Co-IP of V5-tagged SOX6 coexpressed transiently with a Flag-tagged vector, BCL11A-XL (1–835 amino acids), or BCL11A truncation mutants in COS7 cells. Nuclear extracts were immunoprecipitated with an anti-Flag antibody, and copurified proteins were analyzed by Western blot with an anti-V5 antibody. Inputs (10%) are shown. (G) Co-IP of V5-tagged GATA1, GATA2, MTA2, or HA-tagged FOG1 coexpressed transiently with a Flag-tagged Vector, BCL11A-XL (1–835 amino acids), or BCL11A truncation mutants in COS7 cells. Co-IP and Western blot were performed as described in F. Inputs (10%) are shown.