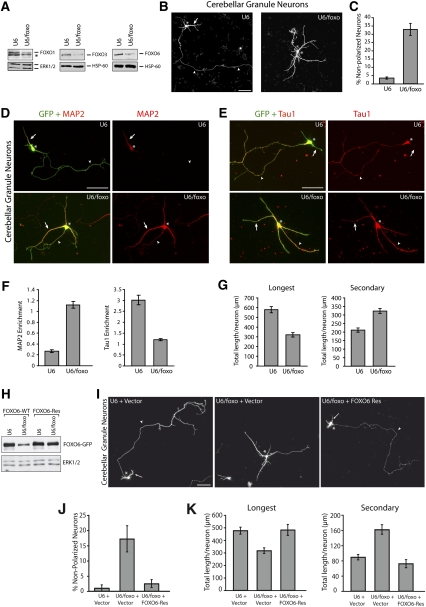
Figure 1.
FOXO transcription factors establish neuronal polarity in cerebellar granule neurons. (A) Granule neurons were electroporated before plating using the Amaxa nucleofection kit with the control U6 or U6/foxo RNAi plasmid. Four days after transfection, lysates were subjected to immunoblotting with a FOXO1, FOXO3, or FOXO6 antibody. FOXO RNAi substantially reduced levels of endogenous FOXO1, FOXO3, and FOXO6 in neurons. The asterisk indicates nonspecific band. (B) Cerebellar granule neurons transfected with the control U6 or U6/foxo RNAi plasmid and a GFP expression plasmid were subjected 4 d after transfection to immunocytochemistry with an antibody to GFP (see Supplemental Fig. 3 for additional lower-magnification panels). Arrows, arrowheads, and asterisks indicate dendrites, axons, and cell body, respectively. Bar, 50 μm. (C) Granule neurons transfected and analyzed as in B were scored as polarized or nonpolarized. FOXO knockdown significantly increased the number of neurons that fail to acquire a polarized morphology (P < 0.01; t-test, n = 3). (D–F) Granule neurons were transfected with the Amaxa electroporation device with the control U6 or U6/foxo RNAi plasmid and the GFP expression plasmid and grown at low density. Five days after transfection, neurons were subjected to immunocytochemistry with the GFP antibody and an antibody to the dendritic marker MAP2 (D) or the axonal marker Tau1 (E). Enrichment of Tau1 and MAP2 was quantified in F. Tau1 and MAP2 enrichment are defined as the intensity of Tau1 or MAP2 immunostaining in the longest neurite divided by the intensity in the second-longest neurite. FOXO knockdown neurons displayed significantly increased MAP2 enrichment (P < 0.001; t-test, n = 3) and significantly reduced Tau1 enrichment (P < 0.01; t-test, n = 3) when compared with control U6-transfected neurons. Arrowheads and arrows point to the longest process and other processes, respectively. Asterisks indicate cell bodies. (G) Morphometric analysis of granule neurons transfected as in B revealed that FOXO RNAi significantly reduced the length of the longest process (axon in control), and concomitantly increased the length of secondary processes (dendrites in control) (P < 0.001; t-test, 213 neurons measured). (H) Lysates of 293T cells transfected with the control U6 or U6/foxo RNAi plasmid together with an expression vector encoding GFP-tagged FOXO6 (FOXO6-WT) or the RNAi-resistant mutant FOXO6 (FOXO6-Res) were subjected to immunoblotting with the GFP antibody (top panel) or an antibody to ERK1/2 (bottom panel). (I–K) Granule neurons transfected with the control U6 or U6/foxo RNAi plasmid, together with the FOXO6-Res expression plasmid or its control vector and an expression plasmid encoding DsRed, were subjected 4 d after transfection to immunocytochemistry with an antibody to DsRed. FOXO6-Res significantly reduced the percentage of nonpolarized neurons in the background of FOXO RNAi (P < 0.01; ANOVA, n = 3). The length of the longest process (axon in control) was significantly reduced and the length of secondary processes (dendrites in control) was significantly increased upon FOXO RNAi (P < 0.001; ANOVA, 200 neurons measured), but not in FOXO6-Res-expressing neurons in the background of FOXO knockdown, when compared with control U6-transfected neurons. Arrows, arrowheads, and asterisks indicate dendrites, axons, and cell body, respectively. Bar, 50 μm.