Fig. 1.
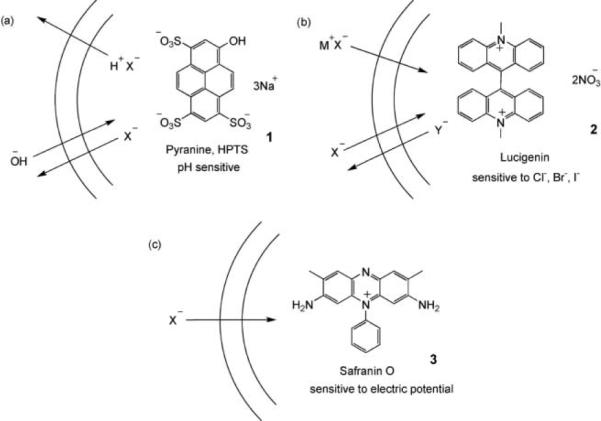
The use of fluorescent dyes to detect anion transport. (a) Pyranine/HPTS: Transport of X− out of the vesicle is accompanied by cotransport of H+ or exchange of OH−. Deprotonation of pyranine (pKa = 7.2) causes an increase in fluorescence when excitation and detection wavelengths are suitably adjusted. (b) Lucigenin: Fluorescence of Lucigenin is quenched by halides (X−), but not by oxoanions such as nitrate, sulfate or phosphate (Y−). The experiment can detect the cotransport of M+X− or exchange of X− and Y−. (c) Safranin O: Fluorescence of the lipophilic, cationic dye increases when it associates with a membrane. Anion transport into the vesicle generates a membrane potential difference (i.e. an electric field) that drives the dye into the membrane, thus increasing fluorescence.
