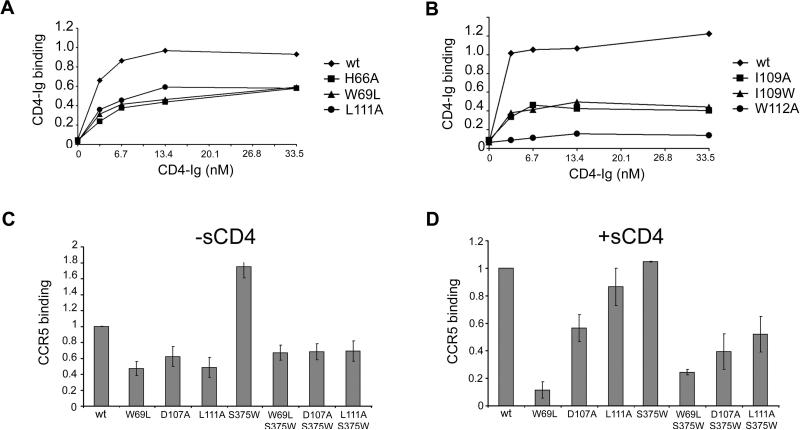
Figure 2. Binding of gp120 receptors.
A, B. The effects of alterations in the Layer 1-Layer 2 interface (A) or in Layer 2 (B) on gp120 recognition by CD4-Ig were examined. Normalized amounts of radiolabeled wild-type mutant gp120 glycoproteins were incubated with increasing concentrations of CD4-Ig for 2 hours at 37°C. The precipitates were washed, run on SDS-polyacrylamide gels, and analyzed by densitometry. All values were normalized to a saturating value for the wt gp120. Representative results from at least two independent experiments are shown. C, D. Similar amounts of radiolabeled gp120 glycoproteins were incubated in the absence (C) or presence (D) of 200 nM sCD4 prior to addition to cells expressing CCR5. After two hours at 37°C, the amount of bound mutant gp120 was determined and normalized to the observed amount of bound wt gp120. Incubation with sCD4 increased the binding of wt gp120 8-fold. The data shown represent the means +/- SEM of two independent experiments.