Abstract
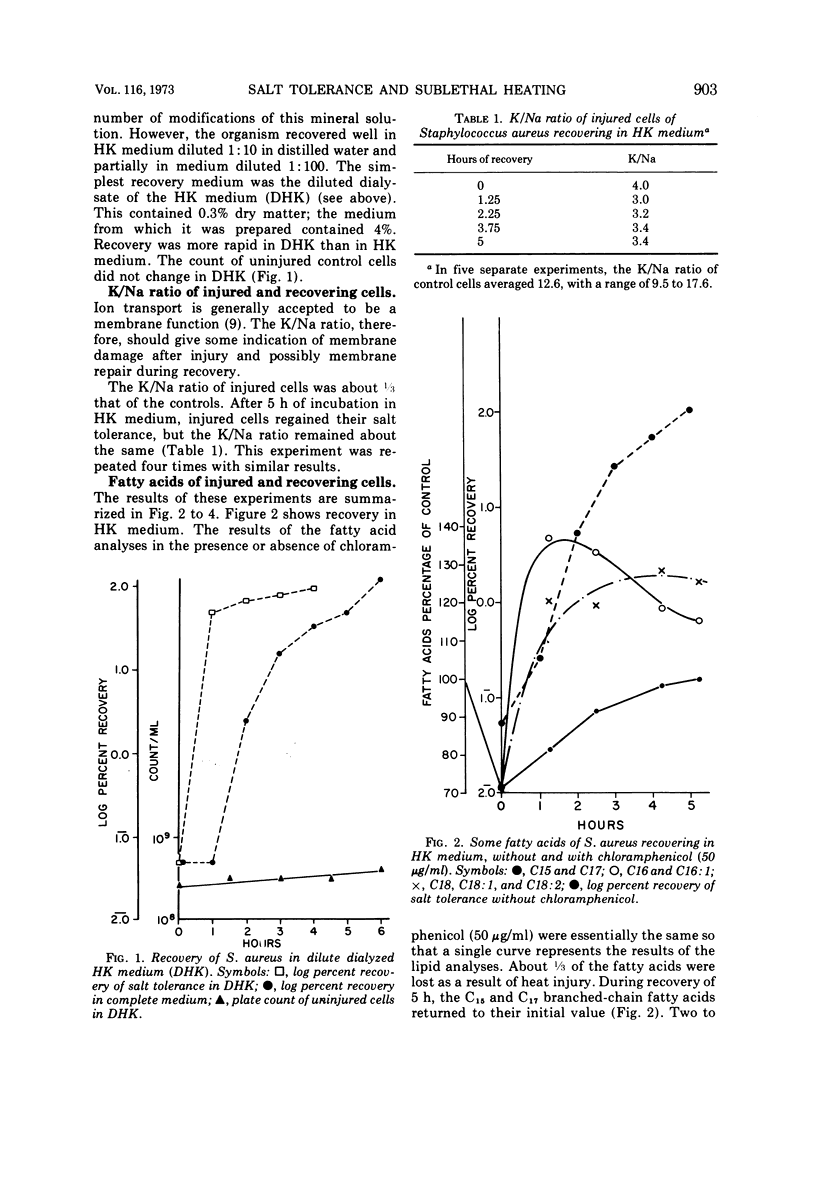
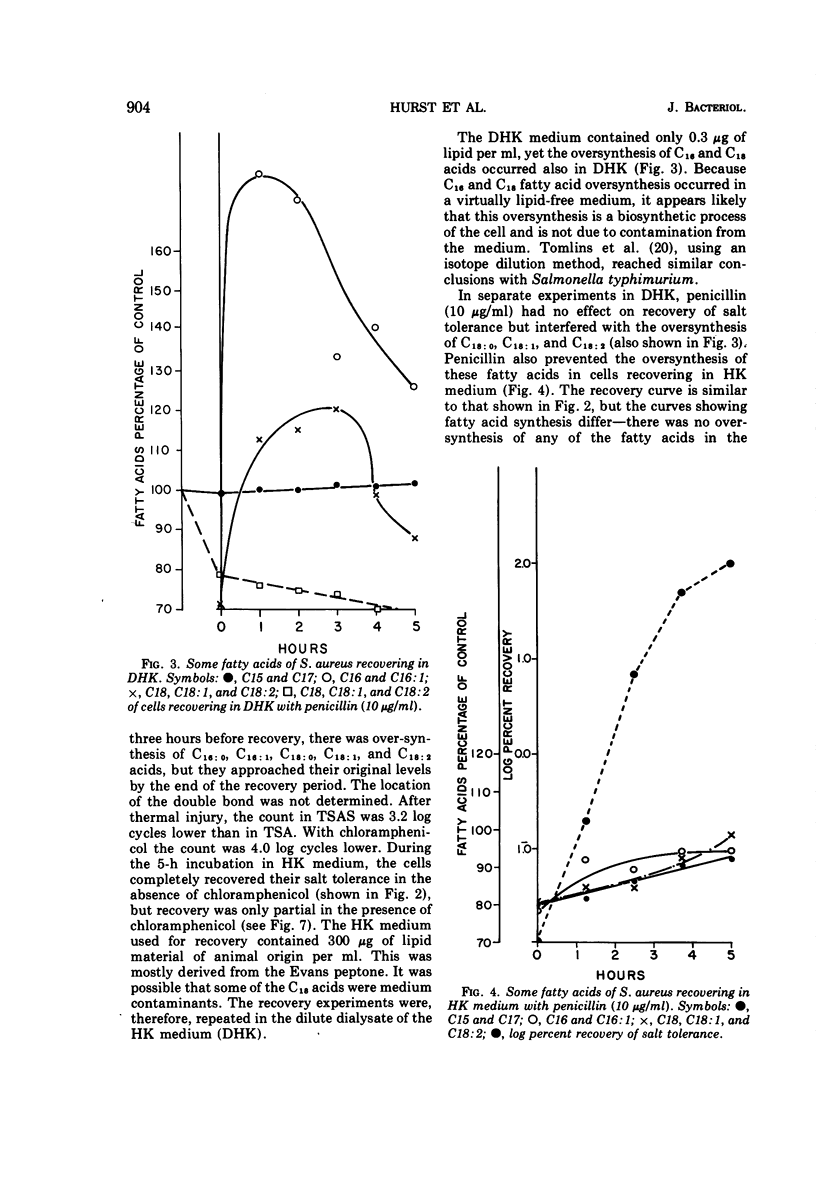
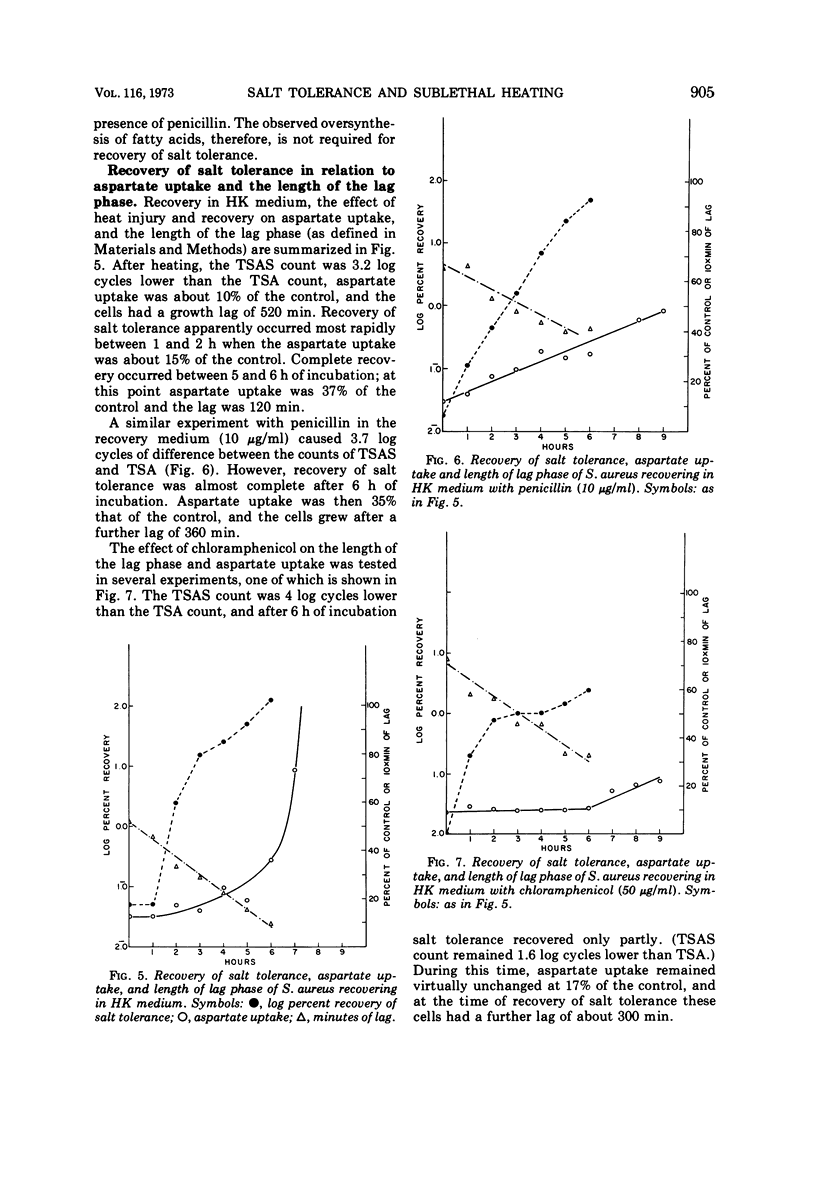
Cultures of S. aureus in 100 mM potassium phosphate buffer heated at 52 C for 15 min lost their tolerance to 7.5% NaCl. After incubation in a complex growth medium or in a diluted dialyzed medium in which unheated cells were unable to grow, salt tolerance was regained. Heat injury caused 30% loss of lipid. During recovery, the concentration of C15 and C17 fatty acids returned to normal, and there appeared to be an oversynthesis of C16 and C18 unsaturated acids. Penicillin abolished the latter reaction without affecting recovery; chloramphenicol did not affect fatty acid oversynthesis but reduced recovery. The K/Na ratio was 12.6 in control cells and 3.4 in injured cells, where it remained during the recovery of salt tolerance. Aspartate uptake was about 10% of the control level after injury and about 35% at recovery. Control cells grew without a lag on subculture, but injured cells which had regained their salt tolerance needed about 2 more h of incubation. Cells recovering with penicillin needed 6 more h, and cells recovering with chloramphenicol did not grow without a prolonged lag. Cells of S. aureus, therefore, may recover their salt tolerance while various membrane functions are still damaged.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allwood M. C. Heat-induced sensitivity to osmotic shock in Escherichia coli and its relationship to cellular potassium content. Microbios. 1971 Sep;4(14):93–96. [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- CHRISTIAN J. H., INGRAM M. The freezing points of bacterial cells in relation to halophilism. J Gen Microbiol. 1959 Feb;20(1):27–31. doi: 10.1099/00221287-20-1-27. [DOI] [PubMed] [Google Scholar]
- Gale E. F. 'Don't talk to me about permeability'. The tenth Marjory Stephenson memorial lecture. J Gen Microbiol. 1971 Sep;68(1):1–14. doi: 10.1099/00221287-68-1-1. [DOI] [PubMed] [Google Scholar]
- Gale E. F., Llewellin J. M. Effect of unsaturated fatty acids on aspartate transport in Staphylococcus aureus and on staphylococcal lipid monolayers. Biochim Biophys Acta. 1971 Mar 9;233(1):237–242. doi: 10.1016/0005-2736(71)90377-4. [DOI] [PubMed] [Google Scholar]
- Gale E. F., Llewellin J. M. Release of lipids from, and their effect on aspartate transport in osmotically shocked Staphylococcus aureus. Biochim Biophys Acta. 1970 Nov 24;222(2):546–549. doi: 10.1016/0304-4165(70)90152-2. [DOI] [PubMed] [Google Scholar]
- Harold F. M. Conservation and transformation of energy by bacterial membranes. Bacteriol Rev. 1972 Jun;36(2):172–230. doi: 10.1128/br.36.2.172-230.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hash J. H. Antibiotic mechanisms. Annu Rev Pharmacol. 1972;12:35–56. doi: 10.1146/annurev.pa.12.040172.000343. [DOI] [PubMed] [Google Scholar]
- Hurst A., Collins-Thompson D. L., Kruse H. Effect of glucose and pH on growth and enterotoxin B synthesis by Staphylococcus aureus, strain S6, after heat injury in sodium or potassium phosphate buffer. Can J Microbiol. 1973 Jul;19(7):823–829. doi: 10.1139/m73-132. [DOI] [PubMed] [Google Scholar]
- Hurst A., Kruse H. Effect of secondary metabolites on the organisms producing them: effect of nisin on Streptococcus lactis and enterotoxin B on Staphylococcus aureus. Antimicrob Agents Chemother. 1972 Mar;1(3):277–279. doi: 10.1128/aac.1.3.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iandolo J. J., Ordal Z. J. Repair of thermal injury of Staphylococcus aureus. J Bacteriol. 1966 Jan;91(1):134–142. doi: 10.1128/jb.91.1.134-142.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kates M. Bacterial lipids. Adv Lipid Res. 1964;2:17–90. [PubMed] [Google Scholar]
- Moats W. A. Kinetics of thermal death of bacteria. J Bacteriol. 1971 Jan;105(1):165–171. doi: 10.1128/jb.105.1.165-171.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STILES M. E., WITTER L. D. THERMAL INACTIVATION, HEAT INJURY, AND RECOVERY OF STAPHYLOCOCCUS AUREUS. J Dairy Sci. 1965 Jun;48:677–681. doi: 10.3168/jds.s0022-0302(65)88321-7. [DOI] [PubMed] [Google Scholar]
- Sogin S. J., Ordal Z. J. Regeneration of ribosomes and ribosomal ribonucleic acid during repair of thermal injury to Staphylococcus. J Bacteriol. 1967 Oct;94(4):1082–1087. doi: 10.1128/jb.94.4.1082-1087.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J., DeVoe I. W. Physiological and morphological effects of phenethyl alcohol upon a gram-negative marine pseudomonad. Can J Microbiol. 1972 Jun;18(6):841–852. doi: 10.1139/m72-130. [DOI] [PubMed] [Google Scholar]
- Tomlins R. I., Vaaler G. L., Ordal Z. J. Lipid biosynthesis during the recovery of Salmonella typhimurium from thermal injury. Can J Microbiol. 1972 Jul;18(7):1015–1021. doi: 10.1139/m72-158. [DOI] [PubMed] [Google Scholar]


