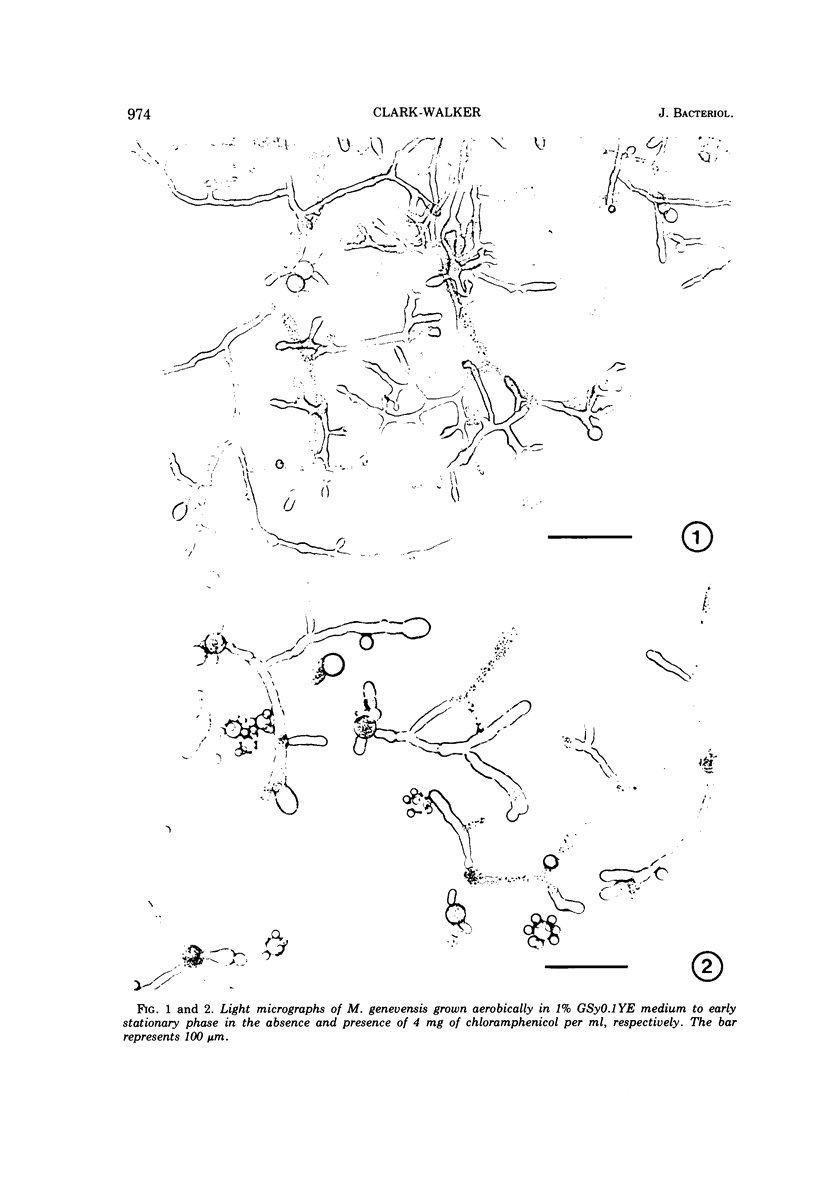
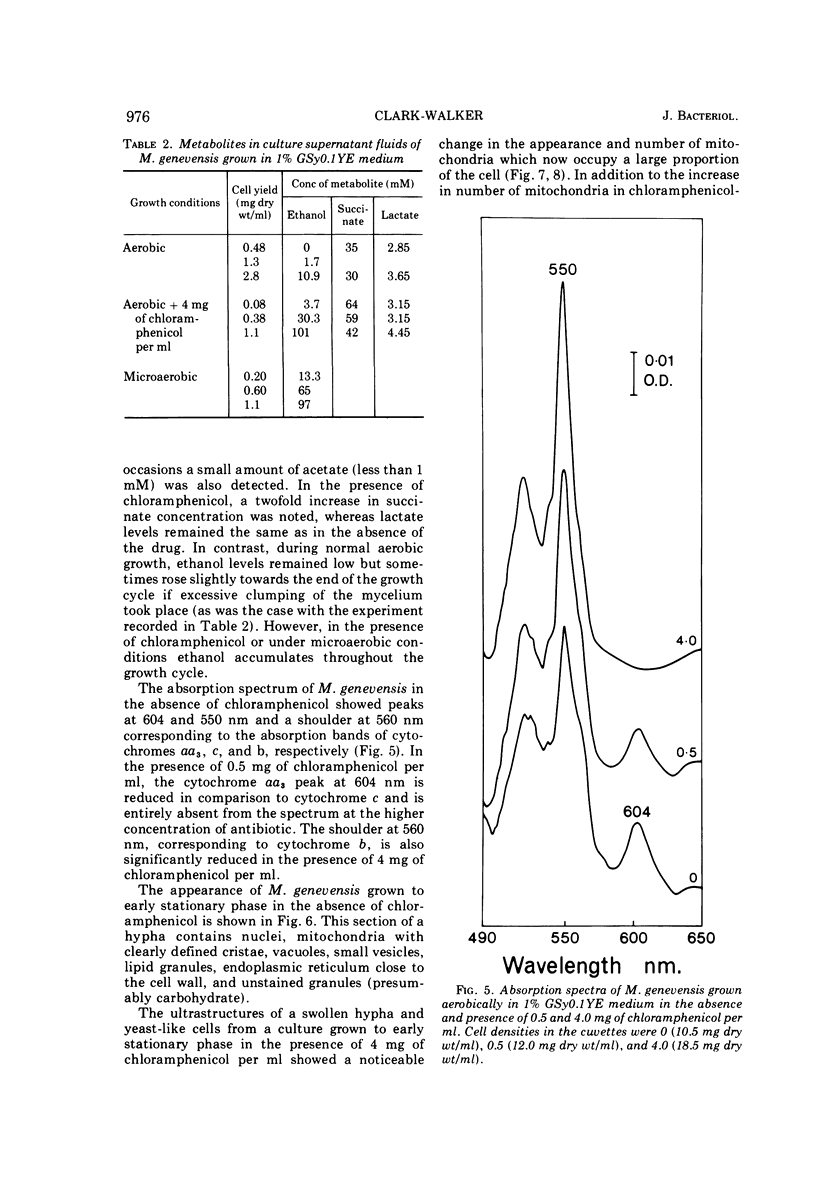
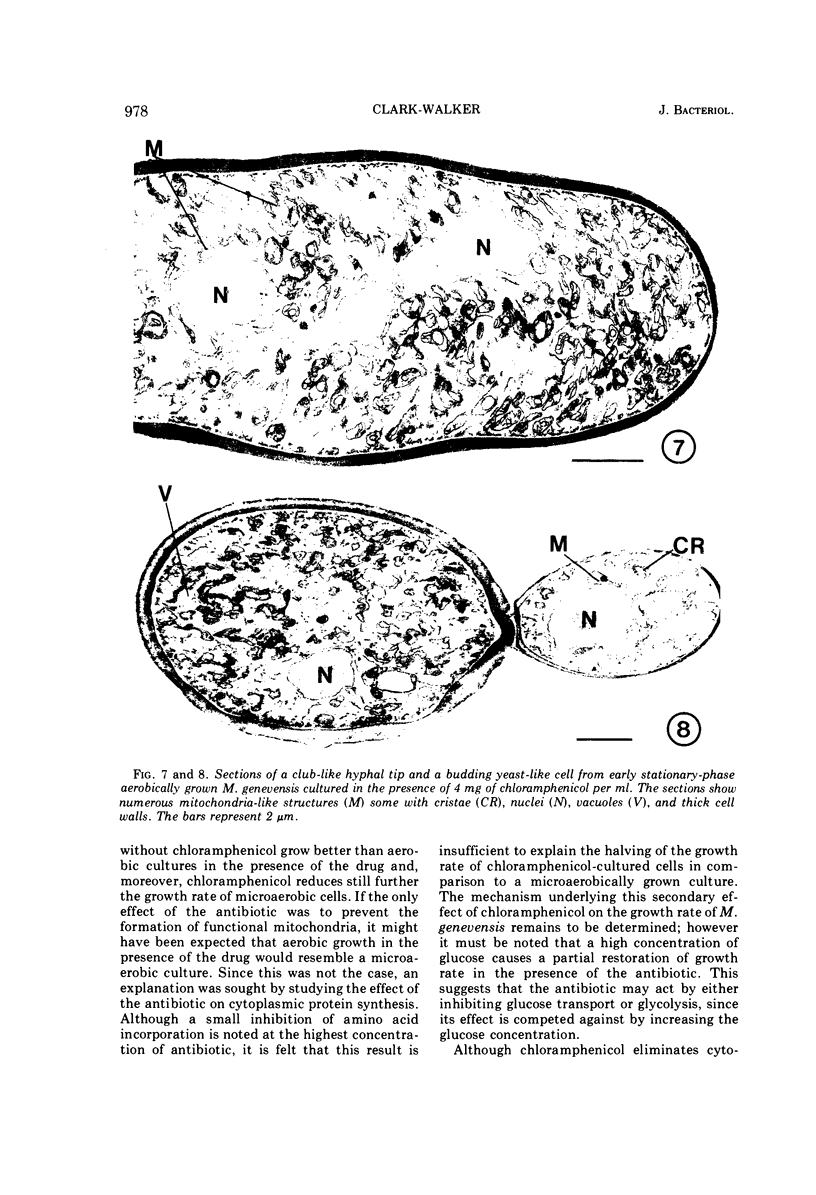
Abstract
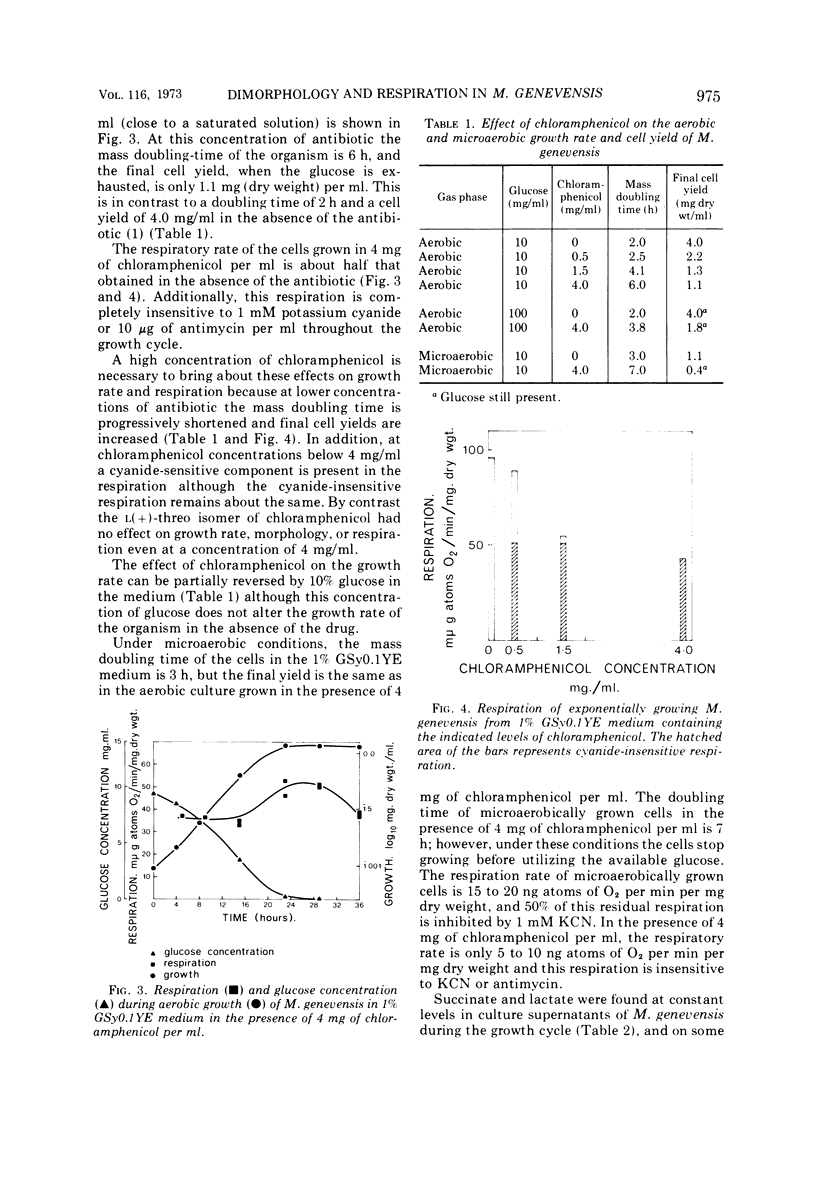
Growth of Mucor genevensis, a facultatively anaerobic dimorphic mold, in high concentrations of chloramphenicol (4 mg/ml) leads to increased numbers of yeast-like cells and small club-like mycelial forms. This change in morphology is accompanied by a threefold increase in the mass doubling time, the loss of cyanide-sensitive respiration, and the development of cyanide-insensitive respiration. Associated with these changes is the absence of cytochromes aa3 and b and the inability of the organism to utilize ethanol; in addition, mitochondria appear more numerous and have less internal membrane. A further inhibitory action of the antibiotic, other than eliminating functional mitochondria, appears likely since microaerobic cultures which lack respiratory ability have twice the mass doubling time in the presence of the drug. Although a small inhibition of amino acid incorporation by cytoplasmic ribosomes is found with a high chloramphenicol concentration, it is insufficient to account for the effect on growth of the microaerobic culture. The nature of this additional effect of chloramphenicol remains to be determined, but it has been shown that increasing the glucose concentration can partially reverse this action of the antibiotic. The effect of the drug on the morphology of the organism is not as dramatic as that of phenethyl alcohol in producing yeast-like forms. However, in view of the action of chloramphenicol in eliminating functional mitochondria in M. genevensis the suggestion that phenethyl alcohol exerts its effect in promoting yeast-like morphology by uncoupling oxidative phosphorylation should be re-examined.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Clark-Walker G. D. Development of respiration and mitochondria in Mucor genevensis after anaerobic growth: absence of glucose repression. J Bacteriol. 1972 Jan;109(1):399–408. doi: 10.1128/jb.109.1.399-408.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark-Walker G. D., Linnane A. W. The biogenesis of mitochondria in Saccharomyces cerevisiae. A comparison between cytoplasmic respiratory-deficient mutant yeast and chlormaphenicol-inhibited wild type cells. J Cell Biol. 1967 Jul;34(1):1–14. doi: 10.1083/jcb.34.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firkin F. C., Linnane A. W. Biogenesis of mitochondria. 8. The effect of chloramphenicol on regenerating rat liver. Exp Cell Res. 1969 Apr;55(1):68–76. doi: 10.1016/0014-4827(69)90457-1. [DOI] [PubMed] [Google Scholar]
- Firkin F. C., Linnane A. W. Differential effects of chloramphenicol on the growth and respiration of mammalian cells. Biochem Biophys Res Commun. 1968 Aug 13;32(3):398–402. doi: 10.1016/0006-291x(68)90674-8. [DOI] [PubMed] [Google Scholar]
- Gordon P. A., Stewart P. R., Clark-Walker G. D. Fatty acid and sterol composition of Mucor genevensis in relation to dimorphism and anaerobic growth. J Bacteriol. 1971 Jul;107(1):114–120. doi: 10.1128/jb.107.1.114-120.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb A. J., Clark-Walker G. D., Linnane A. W. The biogenesis of mitochondria. 4. The differentiation of mitochondrial and cytoplasmic protein synthesizing systems in vitro by antibiotics. Biochim Biophys Acta. 1968 Jul 23;161(2):415–427. [PubMed] [Google Scholar]
- Lloyd D., Evans D. A., Venables S. E. The effect of chloramphenicol on growth and mitochondrial function of the glagellate Polytomella caeca. J Gen Microbiol. 1970 Apr;61(1):33–41. doi: 10.1099/00221287-61-1-33. [DOI] [PubMed] [Google Scholar]
- Marchant R., Smith D. G. The effect of chloramphenicol on growth and mitochondrial structure of Pythium ultimum. J Gen Microbiol. 1968 Mar;50(3):391–397. doi: 10.1099/00221287-50-3-391. [DOI] [PubMed] [Google Scholar]
- Rouslin W., Schatz G. Interdependence between promitochondrial and cytoplasmic protein synthesis during respiratory adaptation in baker's yeast. Biochem Biophys Res Commun. 1969 Dec 4;37(6):1002–1007. doi: 10.1016/0006-291x(69)90231-9. [DOI] [PubMed] [Google Scholar]
- Smith D. G., Marchant R. Chloramphenicol inhibition of Pythium ultimum and Rhodotorula glutinis. Arch Mikrobiol. 1968;60(3):262–274. doi: 10.1007/BF00413493. [DOI] [PubMed] [Google Scholar]
- Terenzi H. F., Storck R. Stimulation of fermentation and yeast-like morphogenesis in Mucor rouxii by phenethyl alcohol. J Bacteriol. 1969 Mar;97(3):1248–1261. doi: 10.1128/jb.97.3.1248-1261.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]