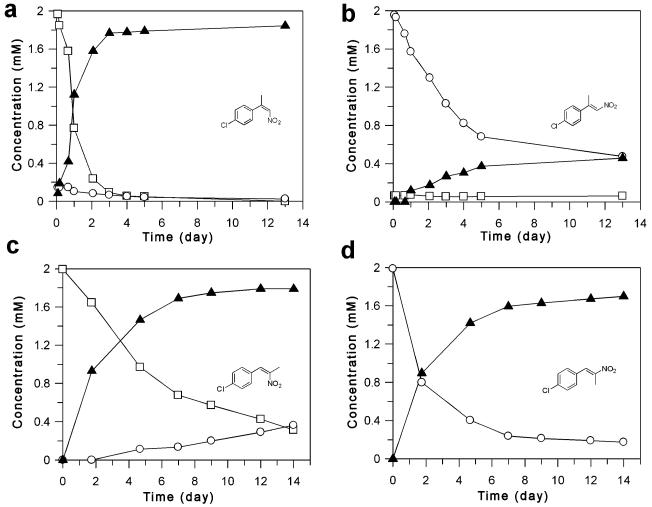
Figure 2.
Time course of the PETN reductase-catalysed reduction of (Z)- and (E)-isomers of 2-(4′-chlorophenyl)-1-nitropropene 4c (a and b, respectively) under biphasic conditions. Substrates: □=(Z)-4c; ○=(E)-4c; product: ▲=(R)-9c. Time course of the PETNR-catalysed reduction of (Z)- and (E)-isomers of 1-(4′-chlorophenyl)-2-nitropropene 5b (c and d, respectively) under biphasic conditions. Substrates: □=(Z)-5b; ○=(E)-5b; product: ▲=(R)-10b. The % ees throughout the reactions were fairly consistent with that of the final product (% ee was 52-60% and 53-56% for (Z)-5a and (E)-5a, respectively) and dropped by only 1% per day.