Table 2.
Reduction of nitroolefins 1-5 by PETN reductase under biphasic reaction conditions[a]
| Number | Substrate | λmax[b] [nm] | Time[c] [days] | Conversion[d],[e] [%] | Yield[d],[e] [%] | Product[f] | ee[e] [%] |
|---|---|---|---|---|---|---|---|
| 1 |
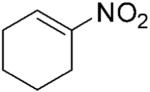
|
264 | 2 | 100 | >99 | 6 | N/A |
| (E)-2 |
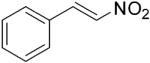
|
319 | 2 | 100[g] | >99[g] | 7 | N/A |
| (E)-3 |
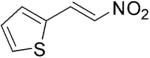
|
357 | 1 | 100 | 82 | 8 | N/A |
| (E)-4a |
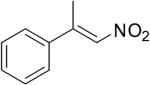
|
306 | 3 | 100[h] | 58[h] | (S)-9a | 89[h] |
| (Z)-4a |
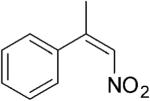
|
208 | 3 | 100[h] | 89[h] | (S)-9a | 96[h] |
| (E)-4b |
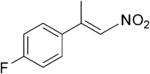
|
312 | 3 | 100 | 86 | (S)-9b | 63 |
| (E)-4c |
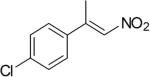
|
310 | 7 | 70 | 18 | (S)-9c | 72 |
| (Z)-4c |
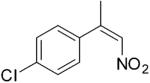
|
221 | 4 | 100 | 91 | (S)-9c | 96 |
| (E)-4d |
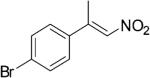
|
314 | 7 | 73 | 35 | (S)-9d | 75 |
| (Z)-4d |
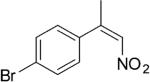
|
224 | 7 | 83 | 76 | (S)-9d | >99 |
| (E)-5a |
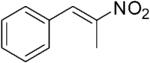
|
316 | 3 | 100[h] | 93[h] | (R)-10a | 14[h] |
| (E)-5b |
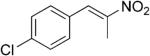
|
321 | 7 | 93 | 84 | (R)-10b | 54 |
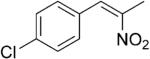
| (Z)-5b |
|
230 | 7 | 88 | 80 | (R)-10b | 60 |
Conditions: The reactions contained potassium phosphate buffer (50 mM KH2PO4/K2HPO4 pH 7.0, 9.2 mL) nitroalkene (1.7 mM in 4.8 mL anaerobic isooctane), NADP+ (20 μM), glucose 6-phosphate (14-20 mM), glucose 6-phosphate dehydrogenase (10 units), deoxygenated PETN reductase (2 μM) and sec-butylbenzene (25 μL). Reactions were agitated at 200 rpm for 1-7 days at 30°C in an anaerobic cabinet. N/A=not applicable.
Maximal absorbance wavelength of the substrate used to monitor reduction.
Time of biphasic reaction in days.
Conversions and yields were determined by HPLC from calibration curve of each standard substrate and products and corrected using sec -butylbenzene as internal standard.
Chiralcel OD-H.
Absolute configuration assigned by comparison with literature data.[15]
Phenomenex C-18.
Chiralcel OJ.
