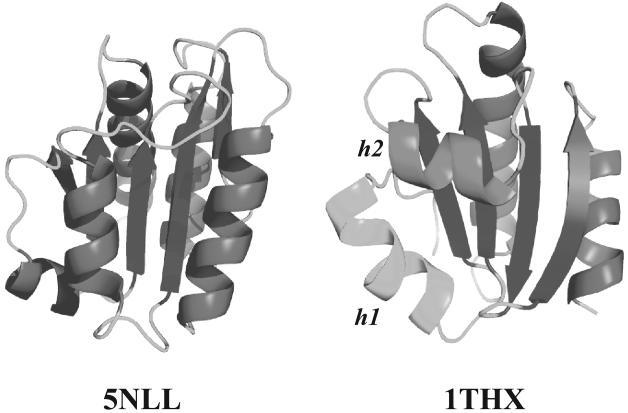
Figure 10. Different packing styles observed in αβ2 motifs in proteins.
5NLL (left panel), abacterial flavodoxin is a small protein of 138 residues formed of a parallel β-sheet with 5 strands surrounded by 5 helices. 5NLL contains 9 αβ2 motifs (8 within the P1 T0 category, and 1 in the P1T1 category), all with parallel strands. In all these motifs, the angle θ is close to 170°. 1THX (right panel), a theoredoxin is another small αβ protein whose structure includes an anti-parallel β-sheet covered with 3 helices, forming 6 αβ2 motifs, two with parallel strands and 4 with antiparallel strands. Note that 1THX contains a fourth helix (shown in green) that does not participate in any of the αβ2 motifs. The three helices present different orientation with respect to the sheet: helix 2 for example (shown in magenta) is perpendicular to the two strands it is packed on. Figure drawn with Pymol.