Abstract
Background
Factors affecting the quality of life (QOL) may be different between young and old stroke patients. However, these issues have not yet been properly investigated.
Methods
We identified 170 young-onset stroke patients (onset between 15 and 45 years of age) who were admitted to the Asan Medical Center. Three hundred and forty follow-up period matched, old-onset stroke patients (onset >45 years of age) were chosen as a control group. A follow-up interview was performed 1~5 years after the onset of stroke in 96 young patients and 160 old patients. With the use of standardized questionnaire, we assessed physical disabilities, activity of daily living (Barthel Index Score, modified Rankin scale), the presence of depression (using DSM IV criteria and Beck Depression Inventory) and socio-economic/job status. The QOL was assessed using the Stroke Specific QOL developed by Williams et al.
Results
The QOL scores were significantly higher in young patients than in old ones. Univariate analysis showed that factors related to low QOL included unemployment, motor impairment, aphasia, dysarthria, dysaphagia and severe modified Rankin score in young patients while poor economic status, unemployment, supratentorial (vs. infratentorial) stroke, anterior (vs. posterior) circulation stroke, the presence of diabetes mellitus, motor impairment, aphasia, dysarthria, dysphagia, visual field defect, severe modified Rankin score, the presence of post-stroke seizures and depression were related to the low QOL in old patients. Cigarette smoking (in old patients) and alcohol drinking (in both young and old patients) were related to high QOL. Multiple regression analysis showed that modified Rankin score was the most important factor explaining low QOL in both groups, while other important factors included depression, visual field defect and anterior circulation stroke in old patients, and the motor dysfunction and dysarthria in young patients.
Conclusions
We conclude that aside from modified Rankin scale, factors affecting the quality of life are different between these two groups. Recognition of these differences may allow us to develop different strategies to improve the quality of life in stroke patients.
Keywords: Cerebrovascular disease, Quality of life, Young, Age
INTRODUCTION
Stroke is a leading cause of death and frequently reduces the level of quality of life (QOL) of the survivors. Many factors have been shown to influence the QOL of these patients, which include motor impairment,1-3 physical disability or dependency in activity of daily living (ADL),1,2,4-6 the presence of depression,4,7-12 cognitive impairment,2 speech disturbances1,10 and the location of the lesion.13
One of the important questions not yet adequately addressed is whether there are differences in the factors affecting the QOL between young-onset (<45 years) stroke patients and old-onset patients. Young-onset stroke patients occupy up to 10% of the total stroke patients,14,15 and have diverse etiologies as compared to old-onset patients. It has also been suggested that the long-term functional outcome, and thus the QOL of young-onset patients is different from that of old patients.16,17 Factors affecting the QOL may also be different between these groups, because the life style and life expectations are different. Although a few previous studies investigated the prognosis,18 functional outcome17 or the QOL16 of young-onset patients, no subjects to compare with old-onset patients were included in those studies. In the present study, we attempted to elucidate the factors affecting the post-stroke QOL in young-onset patients and old-onset stroke patients.
MATERIALS AND METHODS
From the stroke registry of Asan Medical Center, we chose 170 consecutive young-onset patients (onset of stroke between ages 15~45 years) who were admitted to the Asan Medical Center between January 1994 and December 1998. All the patients underwent CT/MRI to confirm the presence of stroke. Excluded were the patients 1) with a transient ischemic attack without progression to stroke 2) who had a past history of stroke 3) who had severe heart, liver or renal disease that may considerably influence on the QOL. We also excluded the patients with hemorrhagic stroke since their prognosis and, probably, the QOL are different from those of ischemic stroke.19
For the control population, we chose 340 old-onset patients (who developed cerebral infarction >45 years of age) from our registry who were admitted just before and just after the target young-onset patients. Aside from age, inclusion and exclusion criteria were identical to those in young-onset stroke patients.
At the time of admission, all the patients were examined by at least one of the two stroke neurologists (JSK, SUK). Our stroke registry contains various data such as age, gender, the level of education (expressed by the number of years of schooling), the presence of risk factors and various neurological deficits. A 'cigarette smoker' was designated when a patient was a current smoker while 'alcohol drinker' was defined when a patient was either a binge drinker or a habitual drinker (more than three times a week).20 In addition, modified Rankin score at discharge, presumed pathogenesis of stroke modified from TOAST15 were recorded. Motor impairment was recorded with the use of 0-V Medical Research Council motor scale. For statistical comparison, motor dysfunction was divided into severe (≤III/V), mild (IV/V) and not impaired, and modified Rankin score was categorized as severe (4-3) and mild (2-0).
Follow up assessment was done between June and August of 2000, and the follow up period ranged from 1 to 5 years in both groups. We first made a telephone call to the patients to obtain a verbal consent for the interview. If they agreed, patients were asked either to visit the outpatient clinic or, if they were unable to attend, to allow a home visit interview. The interview and examination were performed by two trained research nurses with the use of a structured questionnaire regarding the presence of neurological deficit (motor impairment, sensory symptoms, aphasia, dysarthria, dysphagia, visual field defect), depression, and the QOL. The dependency in activity of daily living (ADL) was recorded with the modified Rankin scale and Barthel index score. For statistical analysis, Barthel index score was divided into ≥95, and <95 since patients with score ≥95 were impaired in daily activities.
Post-stroke depression was considered to be present if the patients met either the major depression criteria of DSM-IV or had a score >13 in Beck Depression Inventory.21 Current economic status was divided into good (monthly income >2 million Korean won), average (1~2 million), and poor (<1 million won).22 Current employment status following stroke was also recorded. The interview was done mostly with the patients, and with the relatives in seven patients who had severe communication problems (young patients in 2, old patients in 5).
The QOL was assessed with the use of Stroke Specific QOL (SSQOL) that was recently developed for the QOL of stroke patients.23 The validated Korean version of the questionnaires with the appropriate translation process has been used.24 The SSQOL has 12 main subdomains with a total of 49 items: Energy (four items), family roles (eight items), language (seven items), motility (12 items), mood (eight items), personality (four items), self care (eight items), social roles (seven items), thinking (four items), upper extremity function (nine items), vision (four items) and work/productivity (three items).
Statistics
The data were analyzed with descriptive statistics, students' t-tests, Chi square, Cochran-Mantel Haenszel Chi square and ANOVA using a SAS statistical package. ANOVA was then followed by a Scheffe post hoc test. Factors that could significantly influence QOL were identified via multiple regression analysis. By employing stepwise regression analysis, all variables were investigated to screen for those factors which were related to the changes of QOL. Factors which did not meet a 0.15 significance level were excluded.
RESULTS
1. Study subjects
Among 170 young-onset patients, 51 (30%) could not be reached via phone. Among the remaining 119 patients, nine patients (7.6%) had died, and 14 (11.8.%) did not agree to participate. Therefore, we were able to complete the follow-up interview in 96 young patients; 57 visited the outpatients clinic, 32 underwent a home-visit interview, while in seven patients, only a telephone interview was performed because the patients wanted to do so. Among 340 old-onset patients, 117 (34.4%) were not reached via phone. Among the remaining 223 patients, 45 patients (20.2%) had died, 18 (8.1%) did not give us a consent. Therefore, we completed the follow-up interview in 160 old patients; 104 visited the outpatients clinic, 51 underwent a home-visit interview, while in five patients, only a telephone interview was performed.
Thus, 96 young-onset patients and 160 old-onset patients finally became the subject of our study. The percentage of patients lost during follow-up was not different between these two groups. The modified Rankin score at discharge was not different between the patients who were followed (mild in 84%) and those who were not (mild in 92%) in young patients whereas it was different (p<0.01) between the patients who were followed (mild in 86%) and those who were not (mild in 65%) in old patients. In both groups, age was not different between the patients who were followed and those who were not.
2. Initial and follow up data of the patients
1) Initial data
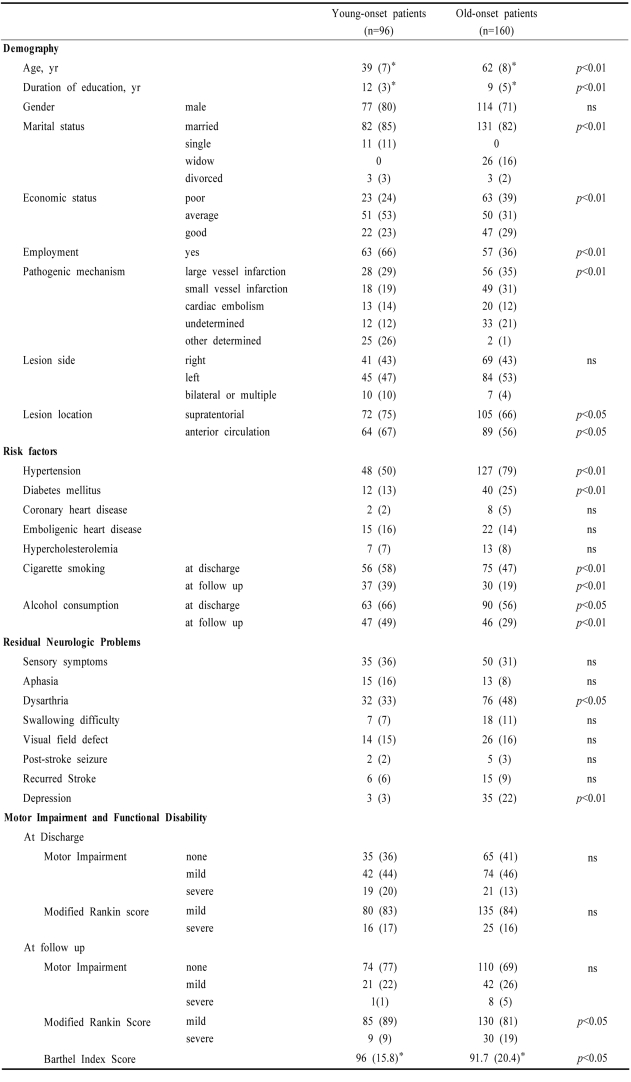
The differences between young-onset patients and old-onset patients are summarized in Table 1. Young patients were more educated, more often lived with their spouses, had better economic and employment status than old ones. They more often had other determined etiology, supratentorial (vs. infratentorial) lesions, and anterior circulation (vs. posterior circulation) stroke. They more often smoked cigarettes and less often had hypertension and diabetes mellitus. Motor impairment and modified Rankin score at discharge were not different between the two groups.
Table 1.
Demographic and clinical features of young-onset stroke patients and old-onset stroke patients
*data presented as 'mean (SD)' while others are presented as number (%), ns; not significant
2) Follow up data
The death rate in old-onset patients (45 patients, 20.2%) was significantly higher (p<0.05) than that of young-onset patients (nine patients, 7.6%). The causes of death were; in young-onset patients: directly stroke-related (herniation, respiratory arrest) in three, infection in two, heart disease in one, malignancy in one, muscular dystrophy in one, unclear in one, while in old-onset patients: directly stroke-related in 10, infection in nine, recurrent stroke in five, malignancy in four, heart/aorta disease in three, suicide in two and unclear in 12. At the time of follow up, young-onset patients more often smoked a cigarette and drank alcohol and less often had dysarthria and post-stroke depression than in old ones. Although the motor impairment at discharge and at the follow up was similar between the two groups, the percentage of the patients who had improvement in motor dysfunction was significantly higher in young-onset patients than in old ones (53% vs. 40%, p<0.05). Functional disability reflected by Barthel index score and modified Rankin score was also significantly better in young-onset patients than in old ones.
3) Factors related to QOL in univariate analysis
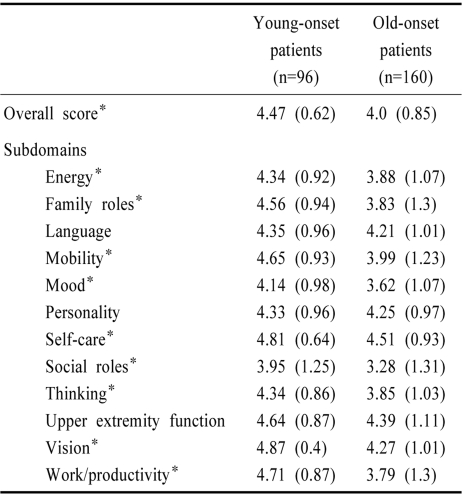
The differences in QOL scores (general as well as each domain) between young-onset patients and old-onset patients are presented in Table 2. The overall QOL score and each domain of SSQOL were significantly higher in young patients than in old ones although differences in domains for language, personality and upper extremity function did not reach statistical significance. When old-onset patients were further subdivided into relatively young (46~65 yr) and relatively old (>65 yr), the QOL score of the latter was significantly lower (mean, 3.8) than that of the former (mean, 4.1).
Table 2.
Quality of life (QOL) score in young-onset stroke patients and old-onset stroke patients
Data presented as mean (SD), *p<0.05
On univariate analysis, factors associated with the low overall QOL in young-onset patients included severe modified Rankin score (both at discharge and at the time of follow up), the presence of motor impairment, aphasia, dysarthria, dysaphagia and no employment status, while alcohol drinking at the time of follow up was related to high QOL. In old-onset patients, factors related to low QOL included motor impairment and severe modified Rankin scale (both at discharge and at the time of follow up), poor economic status, no employment status, large vessel disease (vs. small vessel disease), supratentorial (vs. infratentorial) stroke, anterior (vs. posterior) circulation stroke, the presence of diabetes mellitus, aphasia, dysarthria, dysphagia, visual field defect, post-stroke seizures and depression while smoking and drinking at the time of follow up were related to the high QOL.
4) Factors related to overall and various subdomains of QOL on multiple regression model
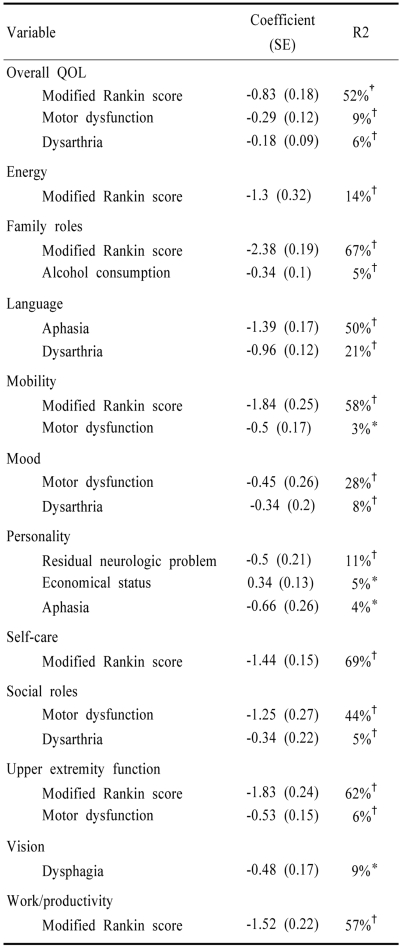
Tables 3 and 4 show the results of multiple regression analysis for the score of overall QOL and that of 12 sub-domains in young-onset stroke patients and old onset patients, respectively. In this analysis, we included data obtained from follow-up examination, which were found to be significant (p<0.05) on univariate analysis. In young-onset patients (Table 3), after exclusion of variables which did not meet a 0.05 significance level, 69 % of the overall variation of QOL scores was explained by the model. The predictors of overall QOL included follow up modified Rankin score, motor dysfunction, dysarthria and economic status. Important (explaining >10% of the variation) factors in each subdomain of QOL included: modified Rankin score for the domains of energy, family role, mobility, self care, upper extremity function and work/productivity; aphasia and dysarthria for the language domain; motor dysfunction for the domains of mood and social role.
Table 3.
Forward stepwise regression model explaining quality of life in young-onset stroke patients
*p<0.05, †p<0.01
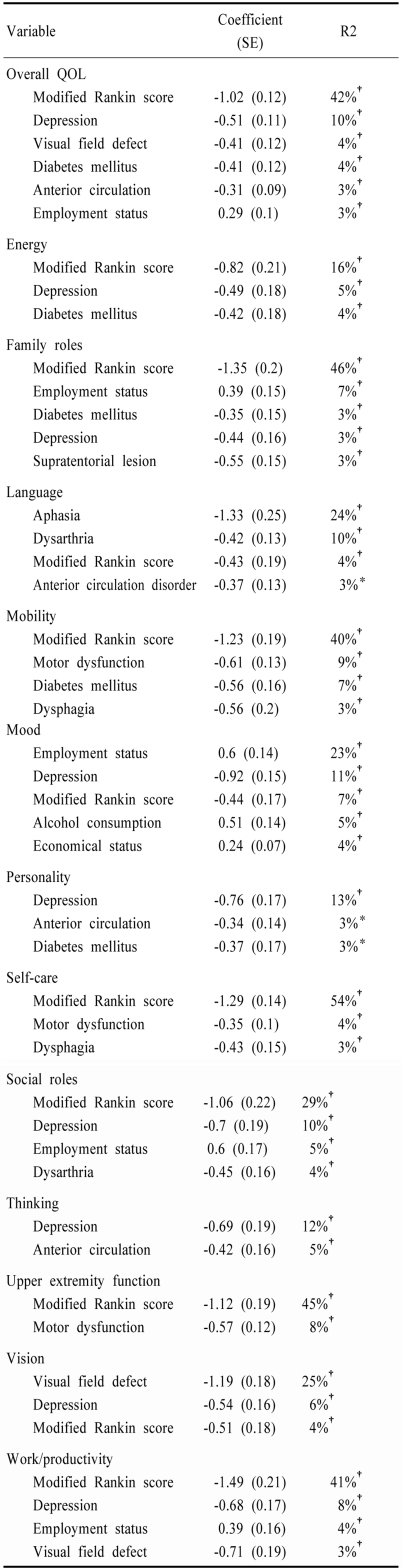
Table 4.
Forward stepwise regression model explaining quality of life in old-onset stroke patients
*p<0.05, †p<0.01
In old-onset patients (Table 4), 67% of the overall variation of QOL scores was explained by the model. The predictors of overall QOL included follow up modified Rankin score, depression, visual field defect, diabetes mellitus, anterior circulation stroke, and current employment status and alcohol consumption. Important (explaining >10% of the variation) factors for each subdomain of QOL included modified Rankin score for the domains of energy, family role, mobility, self care, social roles, upper extremity function and work/productivity; aphasia and dysarthria for the language domain; depression for the domains of mood, personality, and thinking; current employment status for the mood domain; and visual field defect for vision domain.
Because follow-up modified Rankin score, the most important factor influencing the QOL was different between young-onset and old-onset patients, we examined to see whether QOL is still different between the two groups after adjusting for the modified Rankin score. We found that it was significantly different between the two groups after the adjustment (Cochran-Mantel Haenszel Chi square test). Finally, to determine whether there is a confounding effect between age and modified Rankin score, we combined the two groups and ran multiple regression analysis with additional dummy variable (a product of modified Rankin score and age). We found that there was no statistical interaction between age and modified Rankin score.
DISCUSSION
We studied the factors affecting the QOL in both young-onset patients and old-onset patients. Young-onset patients more often smoked cigarettes, and had other determined etiologies and lower follow-up mortality rate, whereas old-onset patients more often had hypertension, diabetes mellitus, and a higher incidence of large vessel disease and small vessel disease. We also found that the frequency and the severity of motor impairment were not different between the two groups either at the time of discharge or at the time of follow up. However, the percentage of the patients who had improvement in motor dysfunction was significantly higher in young patients than in old ones. Consistent with this finding, modified Rankin score was not different at the time of discharge, but was significantly higher in young patients than in old ones at the time of follow-up.
We found that the overall QOL score was higher in young-onset patients than in old-onset ones. In the literature, some inferred that an age is not a factor related to functional prognosis25 while others reported that it is related to a low QOL1,14,26 in stroke patients. These studies, however, did not specifically examine the differences in QOL between young and old patients. As was expected, neurological deficits such as motor dysfunction, aphasia, dysarthria, dysphagia and the overall disability score reflected by modified Rankin score were factors related to QOL in both young and old-onset patients. Multiple logistic regression model showed that modified Rankin score was the most important factor explaining the overall level of QOL as well as domains such as energy, family roles, mobility, self care, upper extremity function and work/productivity in both groups. When modified Rankin score was substituted with Barthel index score, an identical result was obtained.
Of interest was that while motor dysfunction at the time of follow up affected the mobility and self care domains in old-onset patients, it influenced mood/social role domains in young-onset patients. Moreover, the presence of dysarthria was also related to mood/social roles domains in young-onset patients. Thus, as compared to old patients, remaining physical disability seems to be closely related to mood/social roles rather than physical dimensions in young patients. Although this observation may be related to a relatively good recovery of neurological disability and mild Rankin score in the young-onset patients, it may reflect the differences in the social role. As compared to old ones, young patients had a higher employment rate and were more open to society, and the physical disability may therefore more easily disturb their mood and social roles.
In agreement with previous studies,1,3,4,7-12 depression was a factor closely related to QOL in old-onset patients explaining 10% of variance of overall QOL score. It also was an important factor explaining mood, personality, social role, thinking and work/productivity subdomains. However, this was not the case with young-onset patients, probably because the frequency of post-stroke depression was very low in these patients. In addition, the presence of diabetes mellitus was related to low QOL in old-onset patients but not in young patients. The difference may be caused by a longer duration of the illness and probably higher incidence of diabetic complications although the presence of specific complications was not assessed in our study.
As was expected, aphasia was a factor affecting the QOL, especially the language domain in both young and old-onset patients. However, lesion laterality was not a factor related to QOL in both groups. Further analysis after the exclusion of infratentorial strokes produced an identical result (data not shown). This rather contradictory finding may be explained by the possible presence of hemineglect or impaired awareness of the disease in patients with righted-sided lesions, which could have erased the effect of left-sided lesions on lowering the QOL level. Similar interpretation was addressed by a previous study,13 which revealed that patients with right-sided lesions had worse QOL than those with left-sided lesions despite the absence of speech problems.
In this study, we found that young-onset patients less often stopped smoking or drinking after having strokes than old patients did, suggesting that the former were less compliant to medical advice than the latter. Surprisingly, at the time of follow-up, smokers (vs. non-smokers) in old patients and alcohol drinkers (vs. non-drinkers) in both young and old patients had higher QOL scores. The interpretation of this observation should be made cautiously because the high score of QOL in smokers and alcohol users may be the result rather than the cause. Indeed, the patients who smoked or drank had a higher Barthel Index score than who did not (p<0.05, each), which possibly made them less compliant to medical advice.
Finally, our study has limitations. First, there was a high drop out rate in both young-onset patients and old-onset patients. We were unable to reach them by phone because most of our subjects lived in metropolitan Seoul, where many people move frequently for a variety of reasons. To examine these problems, we compared the modified Rankin score at discharge between the patients who were followed and those who were not. In young-onset patients, there was no difference; in old-onset patients, however, the modified Rankin score was significantly higher in the latter than in the former. Considering that the modified Rankin score at discharge was significantly related to QOL, this selection bias may have led us to overestimate the level of QOL in old-onset patients. Nevertheless, this problem did not influence our results that old-onset patients have a lower level of post-stroke QOL than in young patients. Moreover, our primary aim was to elucidate the factors affecting the QOL rather than comparing the level of QOL. Second, although our subjects were follow-up period matched, the large variability in the follow-up period may have affected the QOL in our study population. However, we found that the follow-up period was not related to QOL in both groups. Third, the interview was done with the caregivers when the patients had communication problems. It, however, is unlikely that this limitation affected our results since the case numbers were negligible. Fourth, in our study we did not consider the social support perceived by a patient, which has been shown to be one of the factors related to QOL in previous studies.6,10,27 Finally, in the present study the effect of comorbidity other than vascular risk factors such as degenerative arthritis, heart diseases, cataract etc. on the QOL was not assessed.
Despite these limitations, our results showed that factors affecting the QOL are somewhat different between these groups. Recognition of these differences may allow us to develop different strategies to improve the QOL in stroke patients. For instance, more attention may have to be paid to post-stroke depression, diabetes mellitus or economic status in old patients than in young ones.
Footnotes
This study was supported by research fund from Korean Ministry of Health and Welfare (HMP-00-B-21300-00211).
References
- 1.Niemi ML, Laaksonen R, Kotila M, Waltimo O. Quality of life 4 years after stroke. Stroke. 1989;19:1101–1106. doi: 10.1161/01.str.19.9.1101. [DOI] [PubMed] [Google Scholar]
- 2.Kwa VIH, Limburg M, de Haan RJ. The role of cognitive impairment in the quality of life after ischaemic stroke. J Neurol. 1996;243:599–604. doi: 10.1007/BF00900948. [DOI] [PubMed] [Google Scholar]
- 3.Jonkman EJ, de Weerd AW, Vrijens NLH. Quality of life after a first ischemic stroke: Long-term developments and correlations with changes in neurological deficit, mood and cognitive impairment. Acta Neurol Scand. 1998;98:169–175. doi: 10.1111/j.1600-0404.1998.tb07289.x. [DOI] [PubMed] [Google Scholar]
- 4.Carod-Artal J, Egido JA, González JL, de Seijas EV. Quality of life among stroke survivors evaluated 1 year after stroke: experience of a stroke unit. Stroke. 2000;31:2995–3000. doi: 10.1161/01.str.31.12.2995. [DOI] [PubMed] [Google Scholar]
- 5.Yoon H. Factors affecting quality of life of the Korean aged stroke patients. Int J Agin Hum Dev. 1997;44:167–181. doi: 10.2190/8D0G-4PAW-QH4R-WXU4. [DOI] [PubMed] [Google Scholar]
- 6.Mackenzie AE, Chang AM. Predictors of quality of life following stroke. Disabil Rehabil. 2002;24:259–265. doi: 10.1080/09638380110081805. [DOI] [PubMed] [Google Scholar]
- 7.Ahlsio B, Britton M, Murray V, Theorell T. Disablement and quality of life after stroke. Stroke. 1984;15:886–890. doi: 10.1161/01.str.15.5.886. [DOI] [PubMed] [Google Scholar]
- 8.Parikh RM, Lipsey JR, Robinson RG, Price TR. Two-year longitudinal study of post-stroke mood disorders: dynamic changes in correlates of depression at one and two years. Stroke. 1987;18:579–584. doi: 10.1161/01.str.18.3.579. [DOI] [PubMed] [Google Scholar]
- 9.Astrom M, Asplund K, Astrom T. Psychosocial function and life satisfaction after stroke. Stroke. 1992;23:527–531. doi: 10.1161/01.str.23.4.527. [DOI] [PubMed] [Google Scholar]
- 10.King RB. Quality of life after stroke. Stroke. 1996;27:1467–1472. doi: 10.1161/01.str.27.9.1467. [DOI] [PubMed] [Google Scholar]
- 11.Dorman PJ, Waddell F, Slattery J, Dennis M, Sandercock P. Is the EuroQol a valid measure of health-related quality of life after stroke? Stroke. 1997;28:1867–1882. doi: 10.1161/01.str.28.10.1876. [DOI] [PubMed] [Google Scholar]
- 12.Williams LS, Kroenke K, Weinberger M, Harris LE, Biller J. Post-stroke depression affects quality of life (QOL) but not other stroke outcome measures. Stroke. 1999;30:236. [Google Scholar]
- 13.de Haan RJ, Limburg M, Van der Muelen JHP, Jacobs HM, Aaronson NK. Quality of life after stroke: impact of stroke type and lesion location. Stroke. 1995;26:402–408. doi: 10.1161/01.str.26.3.402. [DOI] [PubMed] [Google Scholar]
- 14.Bogousslavsky J, Pierre P. Ischemic stroke in patients under age 45. Neurol Clin. 1992;10:113–124. [PubMed] [Google Scholar]
- 15.Kwon SU, Kim JS, Lee JH, Lee MC. Ischemic stroke in Korean young adults. Acta Neurol Scand. 2000;101:19–24. doi: 10.1034/j.1600-0404.2000.00004.x. [DOI] [PubMed] [Google Scholar]
- 16.Kappelle LJ, Adams HP, Jr, Heffner ML, Torner JC, Gomez F, Biller J. Prognosis of young adults with ischemic stroke. Stroke. 1994;25:1360–1365. doi: 10.1161/01.str.25.7.1360. [DOI] [PubMed] [Google Scholar]
- 17.Neau J-P, Ingrand P, Mouille-Brachet C, Rosier M-P, Couderq C, Alvarez A, et al. Functional recovery and social outcome after cerebral infarction in young adults. Cerebrovasc Dis. 1998;8:296–302. doi: 10.1159/000015869. [DOI] [PubMed] [Google Scholar]
- 18.Hindfelt B, Nilsson O. Long-term prognosis of ischemic stroke in young adults. Acta Neurol Scand. 1992;86:440–445. doi: 10.1111/j.1600-0404.1992.tb05120.x. [DOI] [PubMed] [Google Scholar]
- 19.Sacco RL. Prognosis of Stroke. In: Ginsberg MD, Bogousslavsky J, editors. Cerebrovascular Disease: Pathophysiology, Diagnosis, and Management. Malden, MA: Blackwell Science; 1998. pp. 879–891. [Google Scholar]
- 20.Kim JS, Yoon SS. Stroke subtypes and risk factors in patients living in southern Seoul, Korea: the impact of hypertension control on stroke subtypes. J Stroke Cerebrovasc Dis. 1998;7:205–210. doi: 10.1016/s1052-3057(98)80009-8. [DOI] [PubMed] [Google Scholar]
- 21.Kim JS, Choi-Kwon S. Poststroke depression and emotional incontinence; correlation with lesion location. Neurology. 2000;54:1805–1810. doi: 10.1212/wnl.54.9.1805. [DOI] [PubMed] [Google Scholar]
- 22.Park K-A, Kim H-S, Kim JS, Kwon SU, Choi-Kwon S. Food intake, frequency, and compliance in stroke patients. Korean J Community Nutrition. 2001;6(3S):542–552. [Google Scholar]
- 23.Williams LS, Weinberger M, Harris L, Clark DO, Biller J. Development of a stroke-specific quality of life scale. Stroke. 1999;30:1362–1369. doi: 10.1161/01.str.30.7.1362. [DOI] [PubMed] [Google Scholar]
- 24.Kim MY. Quality of life and the related factors in cerebrovascualr surgery patients. Seoul, Korea: The graduate school of Seoul National University; 1998. The master's thesis. [Google Scholar]
- 25.Paciaroni M, Arnold P, van Melle G, Bogousslavksy J. Severe disability at hospital discharge in ischemic stroke survivors. Eur Neurol. 2000;43:30–34. doi: 10.1159/000008125. [DOI] [PubMed] [Google Scholar]
- 26.Hackett ML, Duncan JR, Anderson CS, Broad JB, Bonita R. Health-related quality of life among long-term survivors of stroke. Stroke. 2000;31:440–447. doi: 10.1161/01.str.31.2.440. [DOI] [PubMed] [Google Scholar]
- 27.Glass TA, Matchar DB, Belyea M, Feussner JR. Impact of social support on outcome in first stroke. Stroke. 1993;24:64–70. doi: 10.1161/01.str.24.1.64. [DOI] [PubMed] [Google Scholar]