Abstract
We have recently introduced liposome-supported plasmon resonant gold nanoshells (Troutman et al., Adv. Mater. 2008, 20, 2604–2608). These plasmon resonant gold-coated liposomes are degradable into components of a size compatible with renal clearance, potentially enabling their use as multifunctional agents in applications in nanomedicine, including imaging, diagnostics, therapy, and drug delivery. The present research demonstrates that laser illumination at the wavelength matching the plasmon resonance band of a gold-coated liposome leads to the rapid release of encapsulated substances, which can include therapeutic and diagnostic agents. Leakage of encapsulated contents is monitored through the release of self-quenched fluorescein, which provides an increase in fluorescence emission upon release. Moreover, the resonant peak of these gold-coated liposomes is spectrally tunable in the near infrared range by varying the concentration of gold deposited on the surface of liposomes. Varying the plasmon resonant wavelengths of gold-coated liposomes can provide a method for spectrally-coding their light-mediated content release, so that the release event is initiated by the specific wavelength of light used to illuminate the liposomes. The development of spectrally-coded release can find applications in controlled delivery of multiple agents to support complex diagnostic tests and therapeutic interventions.
Keywords: optical imaging, contrast agents, drug delivery, plasmon resonance, controlled release, self assembly, liposomes, nanomedicine
1. INTRODUCTION
Controlled release of therapeutic and diagnostic agents may enable many medical interventions contemplated for the treatment of diseases. As demonstrated thirty years ago, liposomes of certain compositions are capable of thermally controlled release of encapsulated agents. Thermal release occurs at characteristic temperatures corresponding to phase transitions, and is thought to be related to transient changes in lipid membrane ordering at these temperatures, which render the membrane permeable, or leaky, to certain solutes. Perhaps most often studied is membrane permeability occurring at the so called main phase transition (gel-to-liquid crystalline phase), which occurs at 41 °C in a pure DPPC composition. This temperature, which is reasonably close to physiological conditions, can be easily reached under the conditions of a laboratory experiment. However, in clinical applications, local modulation of temperature leading to spatial and temporal control of release is often difficult to achieve.
In comparison, light-controlled release may allow for precise, on-demand content delivery within individual cells in vitro or, in combination with catheter or endoscopic light delivery, may enable precise intervention or diagnostic tests in vivo. While many examples of photochemically induced release from liposomes are described in literature, inherent limitations preclude their clinical applications to date1,2,3,4. Synthesis of plasmon resonant nanoparticles of gold with tunable optical resonances in the visible to near-infrared range5 have been shown to enable controlled release from a polymeric matrix by photothermal conversion, rather than by a photochemical process6. In this work, we apply this principle of photothermal conversion to effect controlled release from thermosensitive liposomes.
We recently introduced degradable plasmon resonant nanoshells created through the deposition of gold onto the surface of liposomes7. Rather than a continuous metallic shell, as demonstrated by others5,8, this composite nanostructure is formed as a shell-shaped array of discrete gold clusters supported by a spherical, metastable core. Its characteristic tunability is different than that of continuous gold nanoshells, and its interpretation incorporates the Maxwell Garnet theory of effective medium to account for the density of distribution of gold clusters on the liposome surface. This material uniquely combines the spectrally tunable optical properties of plasmon resonant coating with the biodegradability and ability to encapsulate and release content afforded by the liposome template.
Here we describe photothermal content release from these biodegradable plasmon resonant nanoshells. We demonstrate that optimal release is obtained when the liposomes are illuminated at the wavelength matching the plasmon resonance peak (on-resonance conditions), and that this release is related to the temperature changes induced by laser illumination. Using microsecond pulses of light, we test the hypothesis that complete content release can be obtained at the temperature corresponding to the so-called pretransition, i.e., a characteristic phase transition occurring at temperatures below the main phase transition, and this illumination regimen is preferred for spectrally selective release.
2. MATERIALS AND METHODS
2.1 Liposome Preparation
Liposomes were prepared from synthetic lipids using a lipid composition similar to one previously demonstrated to exhibit temperature-sensitive controlled release9; the logic supporting this composition is that the instability that occurs during the gel to liquid-crystalline phase transition of lipids sufficiently perturbs the liposome membrane to induce the leakage of contents. The membrane was composed of dipalmitoylphosphatidylcholine (DPPC), monopalmitoylphosphatidylcholine (MPPC), and dipalmitoylphosphatidylethanolamine-[N-methoxy(polyethylene glycol)-2000] (DPPE-PEG2000, all lipids from Avanti Polar Lipids; Alabaster, AL) in a 90:10:4 ratio by weight. MPPC was included to facilitate membrane disruption at the phase transition temperature and DPPE-PEG2000 was included to improve colloidal stability.
The proper proportions of dry lipids were dispersed in chloroform and dried by convection with N2; this process was followed by overnight evaporation under vacuum. Dry lipids were then dispersed in phosphate buffered saline (PBS) containing 5 mM fluorescein to achieve a 60 mM lipid concentration. Liposomes were prepared by the standard freeze-thaw cycle method, which was followed by extrusion through 100 nm polycarbonate membranes, as detailed in previous publications10. Following extrusion, the liposome preparation was dialyzed at 4 °C against PBS to remove excess fluorescein. As determined by quasi-elastic light scattering, the average liposome diameter was 127 nm, intensity weighted (Malvern Zetasizer). All liposome preparations were stored at 4 °C to minimize content leakage.
2.2 Reduction of Gold
The process for the reduction of gold to the surface of liposomes was similar to the technique previously reported7. To summarize, aqueous solutions of gold chloride and of ascorbic acid were prepared at concentrations of 100 mM and 500 mM, respectively. These solutions were added to a 1 mL quantity of the liposome preparation diluted to 20 mM lipids with PBS. For resonance wavelengths matched to the Nd:YAG light source used (1064 nm), for on-resonance illumination, 30 μL of the gold chloride solution was added and gently swirled until uniformly distributed; this was followed by the addition of 45 μL of the ascorbic acid solution and gentle swirling until color, a feature characteristic of the presence of plasmon resonance, developed. The on-resonance preparation demonstrated a broad extinction band centered at 976 nm, with a high level of extinction at 1064 nm. Samples for the off-resonance illumination characterization were prepared in much the same manner, though 12 μL of gold chloride solution and 18 μL of ascorbic acid solution were used to generate a preparation with a resonance peak centered at 610 nm, with minimal extinction at 1064 nm. Following reduction, each sample was individually subjected to a single stage dialysis against PBS at 4 °C to remove any fluorescein that had leaked during the reduction step.
2.3 Extinction Spectra
Extinction spectra were taken with a Cary 5 spectrophotometer on double beam mode against PBS with liposome samples prepared at the same time and in the same manner as those used for the release tests. Samples were diluted to 5 mM lipids to match spectrophotometric range of the instrument.
2.4 Temperature-Induced Content Release
Temperature-induced content release at the pretransition phase was tested for gold-coated and bare (prepared without gold coating) thermosensitive liposomes. A PBS solution was stirred and monitored while was it was gradually heated from 16 °C to 40 °C. Upon reaching the temperature for measurement, 1.97 mL of the PBS was transferred via pipette to a 1 cm (square) path-length cuvette containing 30 μL of the tested liposome solution (maintained at 4 °C). The mixture was briefly incubated before placing it in the fluorescence spectrometer; two incubation periods, 5 seconds and 15 seconds, were tested separately for each temperature measurement. The cuvette holder used in conjunction with the fluorescence spectrometer was maintained at 24 °C to maintain the desired sample temperature and inhibit further release. Volumes were chosen such that the lipid concentration of the test sample was consistently 0.3 mM; this dilution allowed for rapid heating and maintenance of the liposome sample at the desired temperature.
Fluorescence measurements were recorded immediately after incubation over the range of 200 to 800 nm using a diode array spectrometer (Ocean Optics; Dunedin, FL). The excitation source was a 470 nm LED and emission measurements were taken with SpectraSuite Software using a 500 ms integration time. The maximum value of the emission spectrum for each characterized temperature was recorded and the percent release was determined using equation 1:
| (1) |
where I is the maximum intensity of fluorescence emission (generally near 515 nm) for an individually measured sample, I0 is the maximum fluorescence for an untreated or minimum temperature sample, and IT is the fluorescence maximum in the event of complete release, which was determined by replacing 10% of the PBS diluting solution with an aqueous 10% Triton X-100 solution. The rate of release for each temperature point was calculated by dividing the difference in release percent between 15 and 5 second incubation periods by the difference in incubation time.
2.5 Light-Induced Content Release
Content release from plasmon resonant liposomes was then tested by applying a light beam generated by the Nd:YAG laser at 1064 nm (Equilasers, Inc.; Santa Clara, CA) to a liposome sample. A 0.5 mL quantity of liposome solution having a 20 mM lipid concentration was retained in a 1 cm square path-length cuvette at 22 °C, the highest temperature observed to not cause measurable release from the liposome compartments. Light from the Nd:YAG laser was directed toward the sample with a fiber optic cable terminating 1.6 cm above the sample, with a measured half-angle of beam divergence of 7°. Samples were exposed to a number of bursts of laser light. Each burst, or illumination event, lasted 10 seconds, and was made of 200 μs pulses with energy of 41.6 mJ/pulse and repetition rate of 25 Hz. For subsequent fluorescence measurements, 30 μL of the irradiated solution was collected and diluted with 1970 μL of PBS to obtain a 0.3 mM lipid concentration for measurement. Fluorescence measurements were initiated within 30 seconds of illumination. Laser output calibration was performed with a Molectron PM30-VI thermal sensor and PM5200 laser power meter (Coherent; Santa Clara, CA). Sample temperature was continuously monitored during illumination using a K-type wire thermocouple in conjunction with a 4-channel thermometer (Sper Scientific; Scottsdale, AZ). The peak liposome sample temperatures accompanying each illumination event were recorded.
2.6 Content Release at Constant Temperature
Content release rate was examined at two temperatures: 15 °C and 30 °C, near where the pre-transition temperature of the liposome preparations was observed. A 30 μL sample of the on-resonant liposomes was combined with 1970 μL of PBS at the test temperature (15 or 30 °C) and immediately placed in the spectrometer. The cuvette holder of the spectrometer was also set at the test temperature to maintain the temperature of the sample. Fluorescence spectra were collected initially and every 20 s following the addition of PBS for 5 min and the peak emission values at each time point were recorded.
3. RESULTS
The presence of fluorescein is evident in the extinction spectra (Figure 1) by the narrow peak at 485 nm. The gold quantity chosen in each instance generated samples with plasmon resonance maxima that provided either strong or minimal signal at 1064 nm, so that the result of resonance band overlap at the source wavelength could be examined. All samples were prepared and characterized with equal quantities of lipids in solution and, therefore, presumably an equal number of liposomes per unit volume. By the nature of this type of particle, the tuning of the plasmon resonance maxima is dependent on the quantity of gold reduced; consequently, the extinction peak from the 610 nm sample is weaker than that of the 976 nm sample since suspensions were compared on the basis of equal lipid concentration. Like other shell-type structures, these nanoparticles have broad resonance peaks, especially in the infrared.
Fig. 1.
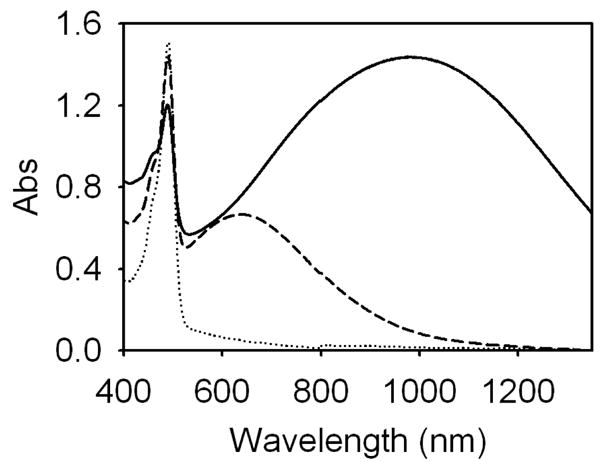
Extinction spectra of liposome preparations: bare liposomes (dotted), gold-coated liposomes resonant at 610 nm (dashed), and gold-coated liposomes resonant at 976 nm (solid). All liposome preparations were loaded with fluorescein.
Figure 2 illustrates liposomal release as a function of temperature. At the temperatures near the pretransition in DPPC bilayers, 35 °C, uncoated liposomes exhibit a release rate more than double that of gold-coated liposomes. Furthermore, uncoated liposomes demonstrate significant increases in release rate at substantially lower temperatures than gold-coated liposomes; whereas bare liposomes show significant increases in release rate around 24 °C, the release rate of gold-coated liposomes rises around 28 °C.
Fig. 2.
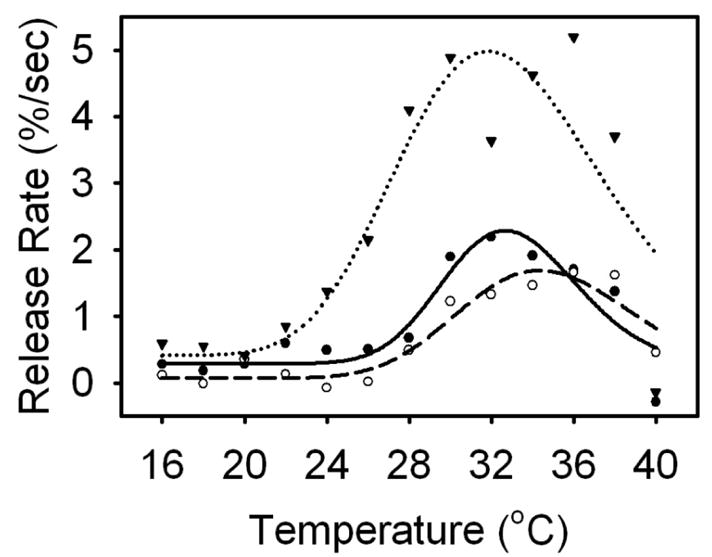
Temperature-mediated release rates for bare liposomes (inverted triangle/dotted), gold-coated liposomes resonant at 610 nm (open circle/dashed), and gold-coated liposomes resonant at 976 nm (closed circle/solid). Data and curve fits are shown. The temperature-mediated release rates for each liposome was modeled using a Gaussian function. The pretransition phase occurred around 32 °C, with bare liposomes initiating release around 24 °C and gold-coated liposomes initiating release around 28 °C.
The sensitivity of each type of liposome preparation to incident laser light is shown in Figure 3. Gold-coated liposomes having a plasmon resonance peak coincident with the illumination source start to experience significant release (~14%) after only 2 illumination events; release from subsequent events follows a saturable trend, with full release occurring after 6 illumination events. Liposomes with a plasmon resonance peak that is not coincident with the illumination did not show significant content release over the tested energy range. The behavior of bare liposomes was substantially different, with slow, but steady, release occurring over illumination events 1–7 and a jump in release occurring at event 8.
Fig. 3.
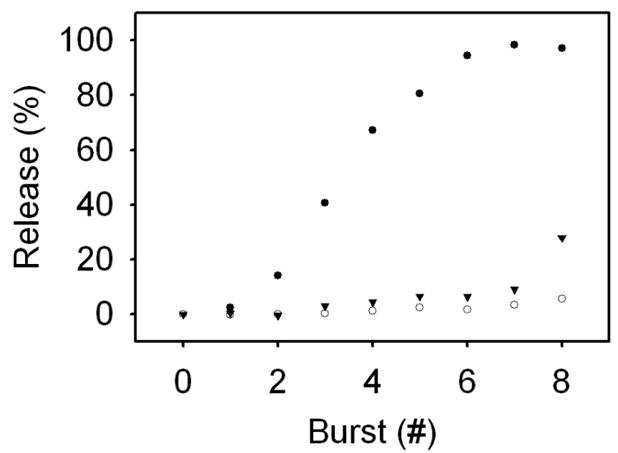
Light-mediated release rates for bare liposomes (inverted triangle), gold-coated liposomes resonant at 610 nm (open circle), and gold-coated liposomes resonant at 976 nm (closed circle). Full content release was observed in gold-coated liposomes resonant at 976 nm after burst 7; at this same point, bare and off-resonant liposomes (resonant at 610 nm) exhibited 3.46 and 9.10% release, respectively. After exposure to 8 laser bursts, bare liposomes had 27.92% content release.
Figure 4 reveals the peak temperature changes accompanying light-induced content release. All three liposome preparations exhibit rapid initial temperature increases, which subsequently level off to apparent steady-state peak temperature values. All preparations reached steady-state temperature values around illumination event 4. However, the peak temperature of liposomes with a resonance peak coincident with the illumination source rose to a significantly higher value before leveling off at over 30 °C than the gold-coated liposomes illuminated off-resonance or the bare liposomes, which both leveled off below 24 °C. The off-resonant gold-coated liposomes and bare liposomes exhibit similar temperature profiles.
Fig. 4.
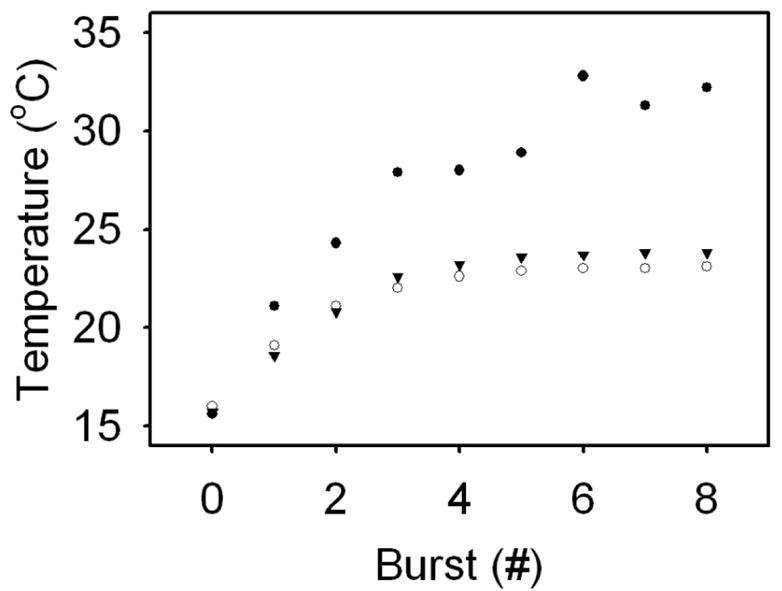
Changes in peak temperature coincident with laser illumination for bare liposomes (inverted triangle), gold-coated liposomes resonant at 610 nm (open circle), and gold-coated liposomes resonant at 976 nm (closed circle). The peak temperatures of each liposome preparation appear to level off to steady-state values. Bare liposomes reach a maximum temperature of 23.8 °C, while on-resonant and off-resonant gold-coated liposomes reach peak temperatures of 32.8 and 23.1 °C, respectively.
Figure 5 shows content release at constant temperature over time for gold-coated liposomes coincident with the light source. At 30 °C, content release occurs immediately and follows a saturable trend, with 50% content release occurring 1 minute after the onset of release. At 15 °C, no appreciable release occurs over the 5 minute duration.
Fig. 5.
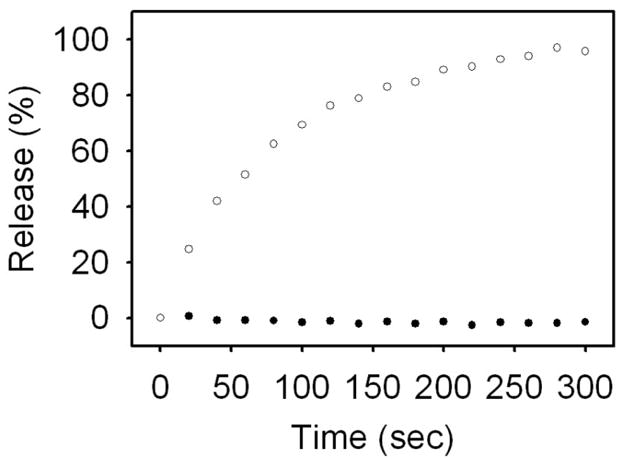
Content release at constant temperature for gold-coated liposomes resonant at 976 nm at 15 °C (closed circle) and 30 °C (open circle). No appreciable content release occurred over 5 minutes at 15 °C, while 50% release occurred after 1 minute at 30 °C.
4. DISCUSSION
Spectrally-coded content release from liposomes may be applied to numerous applications in nanomedicine, including performing complex diagnostic tests and therapeutic interventions requiring the sequential delivery of agents. Novel liposome membrane compositions permit rapid content release upon thermal activation. However, thermal activation within living tissues lacks spatial resolution and is limited by overall physiology. The use of plasmon resonant nanoparticles provides a means by which a temporally controlled light stimulus can be converted into thermal changes with the high spatial resolution characteristic of optical signal delivery. We recently reported that liposome and plasmon resonant structure can be combined into an integrated, biodegradable material and that the plasmon resonant structure of this material may be designed to strongly absorb near infrared radiation, a preferred spectral range for irradiation due to its weak interaction with biological tissue. In this report, we employed the previously developed, spectrally-tunable liposome structure to demonstrate spectrally-coded content release, i.e., content release initiated only in liposomes with a plasmon resonance peak corresponding to the wavelength of the light source.
The temperature-based permeability seemingly associated with the pretransition phase of this liposome composition occurs around 32 °C. This observed release temperature is slightly lower than the pretransition temperature reported for bilayers composed of pure DPPC, 35.3 °C11. This lower observed temperature is likely facilitated by the inclusion of MPPC in the liposome membrane, since binary mixtures are known to adopt a transition temperature between that of its individual components. The inclusion of MPPC is necessary to create the temperature-dependent plasma membrane permeability that is sensitive to the heat generated when the plasmon resonant particle is illuminated. The increased leakage, or permeability, of membranes at a phase transition is associated with the transient perturbation of the lipid packing at transition temperatures, and transition temperatures can be controlled by careful lipid composition selection. Furthermore, bare liposomes were shown to initiate release at significantly lower temperatures than gold-coated liposomes, an event possibly owning to the ionic interactions between the gold and lipid head group.
Full content release using laser illumination was achieved in plasmon resonant liposomes with a resonance peak coincident with the illumination source. Off-resonant liposomes, on the other hand, did not show significant content release over the tested energy range. This is a marked improvement from our previous study in which we observed a significant gap between the energy required for content release from liposomes spectrally matched to the incident laser light and the much higher energy levels needed to cause release from liposomes not matched to the source12. Despite the considerably larger amount of energy needed to achieve full release in off-resonant liposomes, the aforementioned study did not exhibit high specificity for the release of on-resonant over off-resonant liposomes. More specifically, at energy levels required for full release of on-resonant liposomes, more than 25% of content was released from off-resonant liposomes. Here, we have achieved full release from liposomes illuminated on-resonance at energy levels causing less than 3.5% content release in liposomes illuminated off-resonance, clearly demonstrating the possibility of spectrally addressable content release. This spectrally addressable system may allow a sequential release of multiple agents triggered by different wavelengths of light, particularly within the near-infrared, which enables further penetration into tissues with lower levels of phototoxicity than light of shorter wavelengths.
The interaction of laser pulses with gold-coated liposomes produced measurable thermal changes in the sample volume. Using the concept of thermal confinement, it can be shown that the gold-coated liposomes remain in thermal equilibrium with the entire sample volume and, therefore, that light-induced content release is likely accompanied by global increases in sample temperature13. Here, we note that while bare liposomes and illuminated off-resonance liposomes experience similar thermal effects due to laser illumination, liposomes illuminated on-resonance experience much higher increases in temperature. In the temperature-mediated release experiment, significant release appears to first occur in gold-coated liposomes around 28 °C and in bare liposomes around 24 °C. When exposed to bursts of laser light, the off-resonant and bare liposomes did not surpass 24 °C in peak temperature. In contrast, the on-resonant liposomes reached the temperature of observed release (28 °C) after the third and fourth illumination events, respectively.
From observing temperature increases accompanying illumination events, it appears that the onset of light-induced content release from gold-coated liposomes illuminated on-resonance (Figure 4) occurs at temperatures lower than those determined in temperature-induced release experiments (Figure 2). However, the temperatures read by the thermocouple are likely lower than the actual temperatures experienced by liposomes exposed to laser light. Based on calculations from the known beam divergence, the dimensions of the sample in the cuvette, and the distance between the tip of the optical cable and the top of the sample, an estimated 53% of the sample is illuminated by the laser during each illumination event. The thermocouple was placed away from the illumination path to prevent inaccuracies in temperature measurement caused by direct irradiation of the thermocouple. Accordingly, the recorded sample temperature is likely lower than actual peak sample temperatures due to the diffusion of heat to the rest of the sample and the dissipation of heat to the environment. Therefore, it is probable that the experimentally determined onset temperature of light-mediated content release from gold-coated liposomes illuminated on-resonance underestimates its actual value.
While laser illumination caused the on-resonant liposomes reach and surpass the pretransition temperature, it did not cause them to reach, or even approach, the main transition temperature. Evidently, successive light-induced temperature increases to the pretransition temperature was adequate to induce full content release in the gold-coated liposomes. This corresponds with release data taken at constant temperature (Figure 5); full release at the pretransition temperature occurred after about five minutes, with 50% release occurring after 1 minute. In the light-induced release experiments, each illumination event caused a transient increase in the on-resonant sample temperature. These temperature increases were capable of bringing sample temperature toward the pretransition temperature for many seconds and potentially up to 30 seconds (data not shown). Accordingly, it is reasonable to assume that, despite the low pretransition peak observed in the temperature-mediated release, bringing the on-resonant liposome sample to the pretransition temperature in the light-induced release experiments is sufficient to achieve full release.
Bringing liposome temperature to the main phase transition temperature would appear to be advantageous over utilizing the pretransition temperature to achieve more rapid content release. In a previous study, we were able to increase the sample temperature of on-resonant gold-coated liposomes to the main phase transition temperature by using higher laser energy levels12. However, as previously discussed, these higher energy levels also induced substantial release from off-resonant liposomes and the light-induced release process occurs with poor spectral selectivity. Therefore, for this particular liposome composition, it becomes necessary to utilize lower energy levels, and subsequently the pretransition phase, to attain spectrally selective content release. By varying the liposome membrane composition, it might be possible to avoid or eliminate the pretransition release and utilize the main phase transition to initiate more rapid content release while maintaining high spectral selectivity.
Acknowledgments
This work was supported by Grant K25CA120350 (MR) from the National Institutes of Health.
References
- 1.Kano K, Tanaka Y, Ogmura M, Okahata Y, Kunitake T. Photoresponsive membranes- Regulation of membrane properties by photoreversible cis-trans isomerization of azobenzenes. Chem Lett. 1980;1980:421–424. [Google Scholar]
- 2.Bisby RH, Mead C, Morgan CG. Wavelength-programmed solute release from photosensitive liposomes. Biochem Biophys Res Comm. 2000;276:169–173. doi: 10.1006/bbrc.2000.3456. [DOI] [PubMed] [Google Scholar]
- 3.Thompson DH, Gerasimov OV, Wheeler JJ, Rui Y, Anderson VC. Triggerable plasmalogen liposomes: improvement of system efficiency. Biochim Biophys Acta. 1996;1279:25–34. doi: 10.1016/0005-2736(95)00210-3. [DOI] [PubMed] [Google Scholar]
- 4.Bondurant B, Mueller A, O’Brien DF. Photoinitiated destabilization of sterically stabilized liposomes. Biochim Biophys Acta. 2001;1511:113–122. doi: 10.1016/s0005-2736(00)00388-6. [DOI] [PubMed] [Google Scholar]
- 5.Oldenburg SJ, Averitt RD, Westcott SL, Halas NJ. Nanoengineering of optical resonances. Chemical Physics Letters. 1998;288:243–247. [Google Scholar]
- 6.Sershen SR, Westcott SL, Halas NJ, West JL. Temperature-sensitive polymer-nanoshell composites for photothermally modulated drug delivery. Biomed Mat Res. 2000;51:293–298. doi: 10.1002/1097-4636(20000905)51:3<293::aid-jbm1>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 7.Troutman TS, Barton JK, Romanowski M. Biodegradable plasmon resonant nanoshells. Adv Mater. 2008;20:2604–2608. doi: 10.1002/adma.200703026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu G, Mikhailovsky A, Khant HA, Fu C, Chiu W, Zasadzinski JA. Remotely triggered liposome release by near-infrared light absorption via hollow gold nanoshells. J Am Chem Soc. 2008;130:8175–8177. doi: 10.1021/ja802656d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Needham D, Anyarambhatla G, Kong G, Dewhirst MW. A new temperature-sensitive liposome for use with mild hyperthermia: characterization and testing in a human tumor xenograft model. Cancer Research. 2000;60:1197–1201. [PubMed] [Google Scholar]
- 10.Romanowski M, Zhu XY, Kim K, Hruby VJ, O’Brien DF. Interaction of enkephalin peptides with anionic model membranes. Biochim Biophys Acta. 2002;1558:43–45. doi: 10.1016/s0005-2736(01)00421-7. [DOI] [PubMed] [Google Scholar]
- 11.Mabrey S, Sturtevant JM. Investigation of phase transitions of lipids and lipid mixtures by high sensitivity differential scanning calorimetry. Proc Natl Acad Sci USA. 1976;73:3862–3866. doi: 10.1073/pnas.73.11.3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Troutman TS, Leung SJ, Romanowski M. Light-induced content release from plasmon resonant liposomes. in preparation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jacques SL. Role of tissue optics and pulse duration on tissue effects during high-power laser irradiation. Appl Optics. 1993;32:2447–2454. doi: 10.1364/AO.32.002447. [DOI] [PubMed] [Google Scholar]


