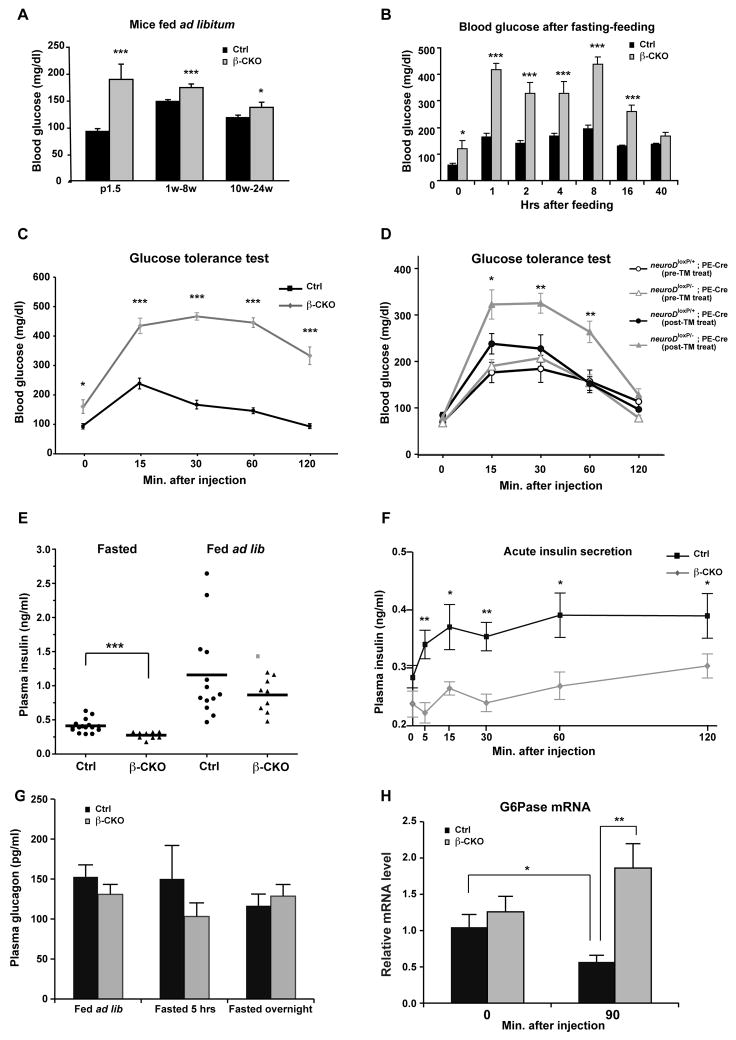
Figure 1. Physiological effects of β cell-specific ablation of neuroD.
(A) Blood glucose levels of neuroD β-CKO and control mice fed ad libitum: P1.5 (n=9–21), 1–8 weeks (n=114–115) and 10–24 weeks (n=34–35). (B) Blood glucose levels of neuroD β-CKO and control mice fasted for 16 h and fed mouse chow (n=9 per genotype). (C) Glucose tolerance test for neuroD β-CKO and control mice (n=10–11 per genotype). (D) Glucose tolerance test for neuroDloxP/−; PE-Cre and neuroDloxP/+; PE-Cre pre-tamoxifen injection and post-tamoxifen injection (n=8–9 each type). The neuroDloxP/−; PE-Cre mice with tamoxifen injection are considered as neuroD PE-CKO. (E) Plasma insulin levels after fasting (16 hours) or fed ad libitum (n=9–16 per genotype). (F) Plasma insulin levels after glucose injection (n=5–7 per genotype).. (G) Plasma glucagon levels fed ad libitum, fasted (5 hours) or fasted (16hours) (n=10–17 per genotype). (H) The expression of G6Pase in control and neuroD β-CKO after fasted for 16 hours (0) and 90 minutes after glucose injection (90′). Values were normalized to β2-microglobin mRNA and expressed as relative to control (n=5 per genotype). * P≤0.05, ** P≤0.01 and *** P≤0.001.