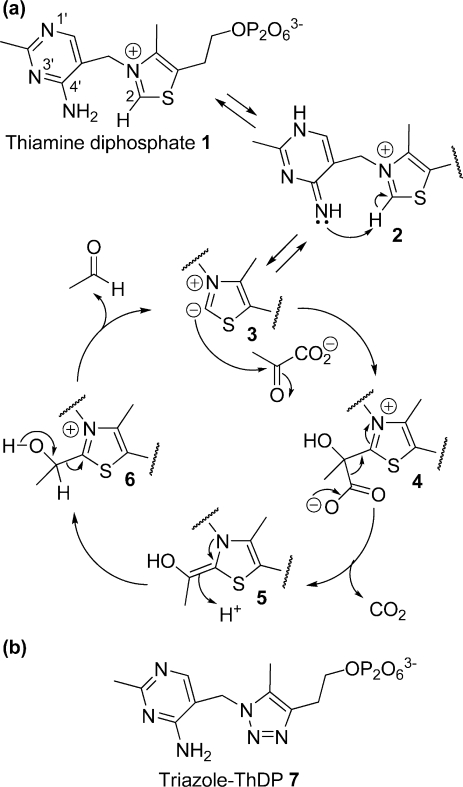
Figure 1.
(a) Catalytic cycle of pyruvate decarboxylation by PDC. Protonation at N-1′ and deprotonation at 4′-NH2 of ThDP cofactor (1) give the imino tautomer (2) which promotes the deprotonation of C2 on the thiazolium ring to form the active ylid (3). The ylid attacks C2 of pyruvate to give the lactyl adduct (4). Decarboxylation then gives the enamine intermediate (5); protonation gives hydroxyethyl-ThDP (6), and then release of the product acetaldehyde regenerates the ylid (3). (b) Structure of the intermediate analogue, triazole-ThDP 7. The C2 and S atoms of ThDP are replaced with nitrogen atoms in the triazole derivative.