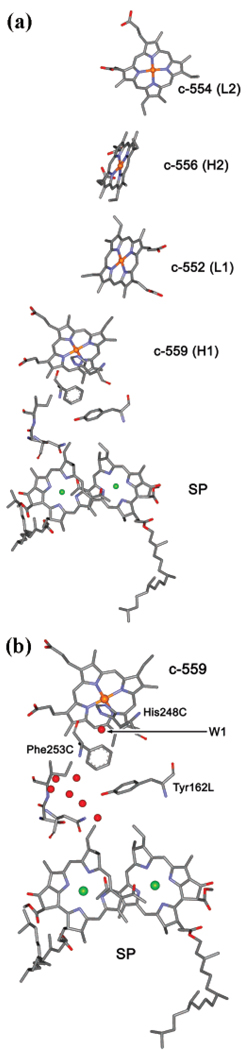
Figure 1.
(a) The structure of redox components of electron-transfer chain discussed in this paper: the special pair (SP) and four hemes. H1 and H2 are two high-potential hemes, L1 and L2 are two low-potential hemes. The electron transfer between the proximal heme, c-559, and oxidized SP is considered in detail in this paper for three redox states of the system: In state 1, c-559 (H1) is reduced; in state 2, c-559 (H1) and c-556 (H2) are reduced; and in state 3, c-559 (H1), c-552 (L1), and c-556 (H2) are reduced prior to oxidation of SP. The actual structure shown is that of the reaction center from Rps. viridis (PDB code, 1PRC), which is believed to be closely related to that of Rps. sulfoviridis studied in this paper. (b) Internal water molecules in Rps. viridis structure between heme c-559 and SP. One water molecule W1 (HOH38) is H bonded to His ligand of heme c-559 and to the backbone of the nearby Phe residue and therefore can have a particularly strongly effect on the ET reaction. Also shown is a small part of the backbone stretching between Phe253C and SP, which might work as a wire for electron tunneling, c-559 → His248C → W1 → Phe253C → backbone → SP, in addition to the most likely ET pathway, c-559 → His248C → W1 → Phe253C → Tyr162L → SP.