FIGURE 1.
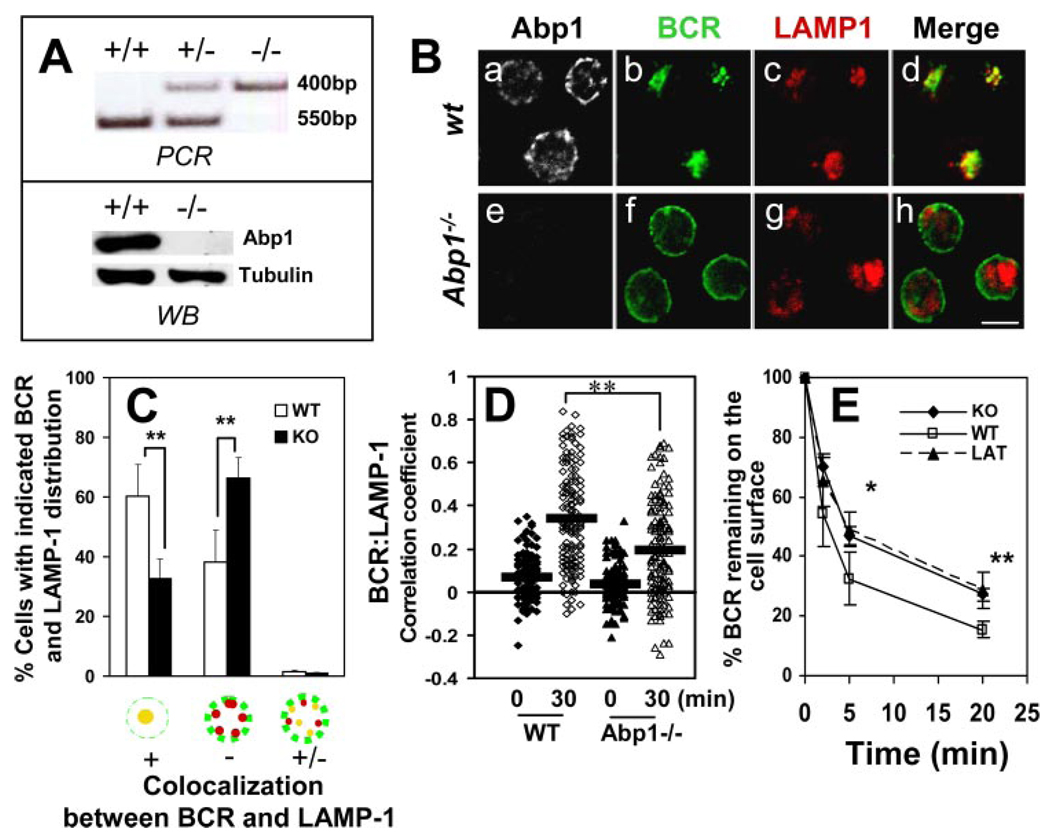
Abp1 gene knockout reduces the rates of BCR internalization and movement from the plasma membrane to late endosomes. A, Abp1 gene knockout in mice was confirmed using PCR (top panel) and Western blot (bottom panel). B, Splenic B cells from wt and Abp1−/− mice were incubated with AF488-F(ab′)2 goat anti-mouse IgM at 4°C for labeling and cross-linking the surface BCR and then chased at 37°C for 30 min. Cells were fixed, permeabilized, and labeled with anti-Abp1, anti-LAMP-1, and fluorochrome-conjugated secondary Abs. Cells were analyzed using a confocal fluorescence microscope. Shown are representative images of three independent experiments. Bar, 5 µm. C, Cells were categorized by visual inspection into three different categories, as follows: cells showing extensive colocalization, no colocalization, and partial colocalization between the BCR and LAMP-1. Cells from more than 10 randomly selected fields containing at least 15 cells per field from three independent experiments were inspected. Shown are the average percentages (±SD) of cells in each of the three categories. **, p < 0.01. D, Shown are correlation coefficients between the staining of the BCR and LAMP-1 in ~100 individual cells of three independent experiments.  , Represent mean correlation coefficients. **, p < 0.0001. E, The surface BCR was labeled with biotin-F(ab′)2 goat anti-mouse IgM at 4°C and chased at 37°C for indicated times. Biotin-anti-mouse IgM left on the surface after the chase was detected by PE-streptavidin and quantified using a FACSCalibur. For latrunculin (LAT) treatment, cells were incubated with 5 µM latrunculin before and during the analysis. The data were plotted as the percentages of the surface-labeled BCR at time 0. Shown are the averages (±SD) of three independent experiments. *, p < 0.05; **, p < 0.01.
, Represent mean correlation coefficients. **, p < 0.0001. E, The surface BCR was labeled with biotin-F(ab′)2 goat anti-mouse IgM at 4°C and chased at 37°C for indicated times. Biotin-anti-mouse IgM left on the surface after the chase was detected by PE-streptavidin and quantified using a FACSCalibur. For latrunculin (LAT) treatment, cells were incubated with 5 µM latrunculin before and during the analysis. The data were plotted as the percentages of the surface-labeled BCR at time 0. Shown are the averages (±SD) of three independent experiments. *, p < 0.05; **, p < 0.01.