Abstract
Trypanosomes evade antibody-mediated lysis via antigenic variation and rapid antibody removal from their cell surface. Recently, in Cell, Engstler et al. (2007) have discovered the mechanism for antibody clearance. Hydrodynamic forces generated by trypanosome swimming create a current, causing surface-bound antibodies to act as “molecular sails.” Consequently, they are swept to the cell posterior, internalized via the flagellar-pocket, and degraded. Hydrodynamic sorting is a novel biological process, possibly applicable in other contexts.
It has long been appreciated that African trypanosomes are a poor target for vaccine development due to their capacity for immune evasion. The dominant mechanism is antigenic variation, whereby a homogeneous variant surface glycoprotein (VSG) coat is expressed on the trypanosome cell surface. The densely packed VSG homodimers prevent access of host antibodies to underlying invariant surface proteins, thus shielding them from the immune response. In a paradigm of antigenic variation, a small proportion of trypanosomes “switch” VSG expression, thereby escaping the lytic immune response directed to the original antigen type (McCulloch, 2004). The basis of the loosely ordered hierarchy of antigen switching is still not fully understood. However, it is not driven by the host immune response but, rather, by distinct VSG gene activation frequencies and by the population dynamics of the two morphological forms of the trypanosome in the bloodstream, slender and stumpy forms (Lythgoe et al., 2007). These differ in proportion during the course of each wave of parasitaemia, with proliferative slender cells giving way to nonproliferative stumpy cells in a density-dependent manner. The slender cells maintain the parasitaemia and provide the source of new antigenic variants, whereas the stumpy forms appear to be adapted for transmission, prolonging their longevity in the face of the developing immune response in order to maximize their opportunity for uptake by the parasite’s vector, the tsetse fly (Matthews, 2005).
In addition to antigenic variation, trypanosomes have a very high rate of endocytosis that allows removal of VSG-bound antibody and thus some evasion of the initial immune response during each wave of parasitaemia. The entire VSG surface coat is recycled through the endocytic system every 12.5 min, with all endocytosis occurring via a specialized organelle called the flagellar pocket, located in the posterior of the cell (Engstler et al., 2004). Laundering of antibody-bound VSG extends the survival time of individual parasites as the antibody response develops by preventing complement activation and formation of the membrane attack complex. However, as the immune response continues to mount, this system is overwhelmed, and cells with antibody-bound VSG are lysed by complement, and only those that have switched survive.
By staining of surface VSG with fluorescent dye, Engstler et al. (2007) developed a method to track antibody clearance from the cell in real time. They found that antibody clearance was rapid (much more so than previously thought) and occurred in three steps, each with different sensitivities to temperature: (1) accumulation of the antibody complex at the posterior of the cell, (2) entry into the flagellar pocket, and (3) endocytosis, whereafter the VSG is recycled and the antibody is degraded. Stumpy cells also cleared antibody more rapidly than slender cells. This matches expectation: it has been known for many years that the stumpy forms are particularly resistant to antibody-mediated lysis (Balber, 1972), such that they survive at least seven times longer at an equivalent antibody titer than slender forms (McLintock et al., 1993). This is proposed to be mediated by their high rate of endocytosis compared to slender forms (though this is debated; Natesan et al., 2007), with bound antibody being rapidly trafficked via their enlarged flagellar pocket.
To further investigate the mechanism of antibody clearance, Engstler et al. (2007) used RNAi-mediated transcript ablation to systematically inactivate trypanosome endocytosis (by targeting clathrin), plasma membrane recycling (by targeting actin), or cell motility. When clathrin was targeted, the inhibition of endocytosis caused bound antibody to accumulate on the surface, such that parasites showed enhanced sensitivity to complement lysis. Nonetheless, posterior accumulation was retained. Similarly, when actin was removed, the parasites retained the ability to redistribute bound immunoglobulin (Ig) and to clear antibody-VSG complexes from their surface. This ruled out antibody “towing” to the cell posterior by plasma membrane recycling or motor proteindriven movement. Most tellingly, however, inhibiting parasite motility by detaching the flagellum from the cell body via ablation of the flagellum adhesion glycoprotein, fla1, caused a loss of antibody-VSG complex sorting to the cell posterior, thereby preventing the first step in antibody clearance. This suggested that it was the action of swimming itself that provided the motive force for the immune complexes to locate to the posterior of the cell, which was then removed by active endocytosis via the flagellar pocket (Figure 1A).
Figure 1. Schematic of Antibody Clearance from the Trypanosome Surface by Hydrodynamic Flow Forces.
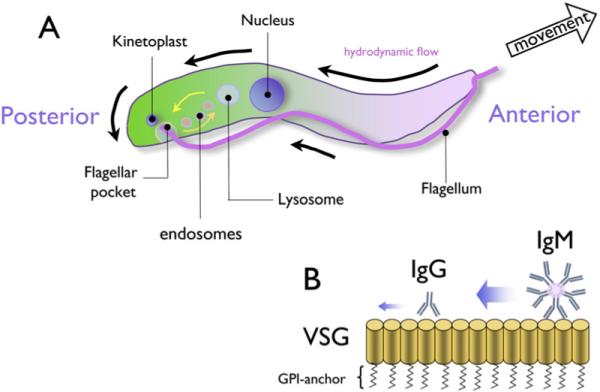
(A) Schematic representation of the African trypanosome. Anterior-directed motion of the parasite generates hydrodynamic flow forces that direct surface-bound antibody toward the posterior of the cell. Here, the VSG-antibody complex is internalized via the flagellar pocket, with antibody being directed to the lysosome, whereas VSG is recycled to the parasite surface. (B) VSG (represented as vertical pillars) is attached to the parasite membrane via a glycosylphosphatidylinositol (GPI) anchor, forming a homogenous coat on the parasite surface. The GPI anchor enables free migration within the lipid bilayer, facilitating molecular flow. Antibodies bind to the VSG and are sorted via hydrodynamic flow forces, with larger molecules (IgM) moving more rapidly than smaller molecules (IgG), as indicated by the relative arrow size.
To challenge their hypothesis, Engstler et al. (2007) targeted an axonemal dynein arm component, DNAI1. DNAI1 ablation has previously been shown to cause the flagellar beat to reverse polarity, causing the trypanosome to swim backward (Branche et al., 2006). Crucially, and consistent with their hypothesis, when the trypanosome reversed direction the antibody-VSG complex accumulated at the opposite, anterior, end of the cell away from the flagellar pocket.
The proposal that physical flow forces caused bound macromolecules to be swept posteriorly on the swimming trypanosome suggested that the size of the bound ligand might affect its rate of migration. Consistent with this, larger ligands (e.g., IgM pentamers versus the IgG monomer) acted as better “sails,” were more prone to “catching” the hydrodynamic forces generated by the cell swimming, and were consequently cleared from the cell more quickly (Engstler et al., 2007) (Figure 1B). Moreover, clearance was more effective in viscous medium such as blood and was predicted to be more rapid in confined spaces, such as capillaries.
Although effective, the protective effect observed is likely short lived: increasing the number of VSG molecules bound with IgG increased the viscosity of the membrane through molecular crowding, consequently decreasing the rate of antibody clearance (Engstler et al., 2007). The biological implications of this are clear: as the immune response becomes stronger, the cell becomes less efficient at dealing with it. Hence, understanding the dynamics of the changes in antibody titer (and isoform) and subsequent clearance during each parasitaemia will be a key factor in establishing just how much benefit this mechanism can be to the trypanosome. In other words, how much time does flow-mediated antibody sorting “buy” the trypanosome in its battle against the immune system? This is important information necessary to evaluate the overall biological significance of the phenomenon to the parasite, where other variables such as VSG switch frequency, differing antibody titers within diverse body compartments, and the potential for parasite-induced immunomodulation or influences on parasite virulence (e.g., Webb et al., 1997) might also play a part. A clear benefit of the present study, however, is its accessibility to further investigation and modeling. For example, the differential flow and clearance of IgG and IgM makes clear predictions for parasite survival when growth in IgM knockout or wild-type mice is compared, with likely additional consequences for the balance of slender and stumpy cells in the resulting parasitaemias.
By demonstrating the concept of using molecular sails to facilitate antibody clearance, the work of Engstler et al. further highlights the elegance of the immune evasion mechanisms employed by African trypanosomes as they balance longevity in the bloodstream with transmissibility to the tsetse fly. Clearly, there is much still to be learned in the complex interplay between the mechanisms of host immunity (including antibody-mediated cellular defenses), parasite immune evasion, density-dependent modulation of the parasitaemia, and transmissibility. It has yet to be determined whether this model can be applied to other systems, but Engstler et al. speculate that flow-forced sorting of proteins could be a general organizing principle in numerous biological contexts.
ACKNOWLEDGMENTS
We thank Judi Allen and Rick Maizels for discussions. S.D.D. is funded by a BBSRC studentship; work in K.R.M.’s laboratory is funded by the Wellcome Trust.
REFERENCES
- Balber AE. Exp. Parasitol. 1972;31:307–319. doi: 10.1016/0014-4894(72)90122-1. [DOI] [PubMed] [Google Scholar]
- Branche C, Kohl L, Toutirais G, Buisson J, Cosson J, Bastin P. J. Cell Sci. 2006;119:3443–3455. doi: 10.1242/jcs.03078. [DOI] [PubMed] [Google Scholar]
- Engstler M, Thilo L, Weise F, Grunfelder CG, Schwarz H, Boshart M, Overath P. J. Cell Sci. 2004;117:1105–1115. doi: 10.1242/jcs.00938. [DOI] [PubMed] [Google Scholar]
- Engstler M, Pfohl T, Herminghaus S, Boshart M, Wiegertjes G, Heddergott N, Overath P. Cell. 2007;131:505–515. doi: 10.1016/j.cell.2007.08.046. [DOI] [PubMed] [Google Scholar]
- Lythgoe KA, Morrison LJ, Read AF, Barry JD. Proc. Natl. Acad. Sci. USA. 2007;104:8095–8100. doi: 10.1073/pnas.0606206104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews KR. J. Cell Sci. 2005;118:283–290. doi: 10.1242/jcs.01649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCulloch R. Trends Parasitol. 2004;20:117–121. doi: 10.1016/j.pt.2003.12.004. [DOI] [PubMed] [Google Scholar]
- McLintock LM, Turner CM, Vickerman K. Parasite Immunol. 1993;15:475–480. doi: 10.1111/j.1365-3024.1993.tb00633.x. [DOI] [PubMed] [Google Scholar]
- Natesan SK, Peacock L, Matthews K, Gibson W, Field MC. Eukaryot. Cell. 2007 doi: 10.1128/EC.00213-07. in press. Published online September 28, 2007. 10.1128/EC.00213-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb H, Carnall N, Vanhamme L, Rolin S, Van Den Abbeele J, Welburn S, Pays E, Carrington M. J. Cell Biol. 1997;139:103–114. doi: 10.1083/jcb.139.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]


