Abstract
Background:
A strategy to reduce the secondary effects of anti-cancer agents is to potentiate the therapeutic effect by their combination. A combination of vitamin K3 (VK3) and ascorbic acid (AA) exhibited an anti-cancer synergistic effect, associated with extracellular production of H2O2 that promoted cell death.
Methods:
The redox-silent vitamin E analogue α-tocopheryl succinate (α-TOS) was used in combination with VK3 and AA to evaluate their effect on prostate cancer cells.
Results:
Prostate cancer cells were sensitive to α-TOS and VK3 treatment, but resistant to AA upto 3.2 mM. When combined, a synergistic effect was found for VK3–AA, whereas α-TOS–VK3 and α-TOS–AA combination showed an antagonist and additive effect, respectively. However, sub-lethal doses of AA–VK3 combination combined with a sub-toxic dose of α-TOS showed to induce efficient cell death that resembles autoschizis. Associated with this cell demise, lipid peroxidation, DNA damage, cytoskeleton alteration, lysosomal–mitochondrial perturbation, and release of cytochrome c without caspase activation were observed. Inhibition of lysosomal proteases did not attenuate cell death induced by the combined agents. Furthermore, cell deaths by apoptosis and autoschizis were detected.
Conclusion:
These finding support the emerging idea that synergistic combinations of some agents can overcome toxicity and other side-effects associated with high doses of single drugs creating the opportunity for therapeutically relevant selectivity.
Keywords: ascorbic acid, vitamin E, vitamin K3, α-tocopheryl succinate, synergism, prostate cancer
Standard cancer therapy is plagued by undesirable adverse side-effects, including toxicity to normal cells and evasion of immune surveillance. These drawbacks have fuelled efforts to develop new anti-cancer agents and additional and/or complementary strategies to inhibit cancer cell growth. These strategies include approaches such as cancer immunotherapy (Lu et al, 2002; Roberts et al, 2002), caloric restriction (Zhu et al, 1997); genetic manipulation (Koch et al, 2000); a concomitant increase of the therapeutic effect of anti-cancer drugs while reducing undesirable secondary effects (Buc-Calderon et al, 1989); or potentiation of the anti-tumour therapeutic effects of drugs by their combination (Uwagawa et al, 2009; Zahorowska et al, 2009; Beauchamp et al, 2009; Eichhorn et al, 2010). Drug combination is a promising strategy to overcome side-effects associated with high doses of single drugs (Keith et al, 2005; Lehár et al, 2009).
The combined action of ascorbic acid (AA) and vitamin K3 (VK3) has been reported to synergistically induce cell death in different cancers (De loecker et al, 1993; Verrax et al, 2005; Tareen et al, 2008). Oxidative stress generated by the ascorbate-driven quinone redox cycling kills tumour cells by a mechanism including glycolysis inhibition, loss of calcium homoeostasis, DNA damage, and altered activity of mitogen-activated protein kinases (MAPK). In this case, cell death involves necrosis rather than apoptosis or macroautophagy (Beck et al, 2009). Pharmacological doses of AA (>0.2 mM) are required to induce oxidation-dependent cytotoxicity both in vitro (Beck et al, 2009) and in vivo (Tareen et al, 2008). Cells convert ascorbate to the ascorbyl radical (A−) with concomitant formation of H2O2 in extracellular fluids (Chen et al, 2007), resulting in toxicity towards tumour cells (Rhee, 2006). However, this approach faces a possible complications in that the therapeutic synergy of the drug combination could be accompanied by deleterious side-effects, and by the possibility that the extracellular levels of H2O2 main drop below 1 μM, at which concentration proliferation rather than cell death may be promoted (Rhee, 2006).
To overcome these potential adverse effects, we have tested the hypothesis that cell death induced by an extracellular oxidative insult could be enhanced by combining apoptotic agents acting through different modes of action. One of the emerging targets for anti-cancer drugs is mitochondria that is central to induction of programmed cell death (Gogvadze et al, 2008; Trachootham et al, 2009). Our previous work has identified mitochondria as transmitters of apoptosis induced by the redox-silent analogue of vitamin E (VE) α-tocopheryl succinate (α-TOS) (Neuzil et al, 2004, 2007a) that is selective for cancer cells (Neuzil et al, 2001a, 2001b) and suppresses tumours in a variety of pre-clinical models (Neuzil et al, 2001b; Stapelberg et al, 2005; Wang et al, 2007; Dong et al, 2008, 2009). This suggests that the VE analogue is a promising anti-cancer drug that may suppress tumours by causing cell death of malignant cells as well as synergizing with other anti-cancer agents. In this study, we used the agent in combination with VK3 and AA stimulating the redox cycling system to evaluate their combined anti-cancer effects on one human prostate cancer cell line.
Materials and methods
Cell culture and reagents
The prostate cancer cells (PC3) and fibroblasts (IMR90) purchased from the American Type Culture Collection were grown in the RPMI-1640 and DMEM medium, respectively, supplemented with 2 mM L-glutamine, 100 U ml−1 penicillin, 100 μg ml−1 streptomycin, and 10% FBS. The cells were cultured at 37°C and 5% CO2 in a humidified incubator. AA (Sigma, St Louis, MO, USA) was dissolved in PBS (pH 7.4) immediately before use. α-TOS and VK3 (menadione) (both from Sigma) were dissolved in ethanol and DMSO, respectively, diluted in complete medium to the final concentration and added to cells at 0.1% of solvent (v/v).
Cytoxicity and isobologram analysis
PC3 and IMR90 cells were plated in 96-well flat-bottom tissue culture plates at 104 per well, allowed to attach overnight and incubated for 24, 48 and 72 h with α-TOS (10–100 μM), VK3 (1–30 μM), and AA (0.1–3.2 mM) alone or in combination. Cell viability was determined using the MTT assay (Carmichael et al, 1987). Briefly, after the exposure of cells, 10 μl of MTT (5 mg ml−1 in PBS) was added and the plate incubated at 37°C for 3 h. After removing the media, 200 μl of isopropanol was added and mixed to dissolve the crystals. Absorbance was read at 550 nm in an ELISA plate reader and control absorbance was designed as 100%. Survival curves were generated and the IC50 values determined. The effect of combination of the drugs in PC3 and IMR90 cells was estimated both by plotting the percentage of dead cells after treatment with the drug alone or in combination with another drug at its IC50 concentration and by the isobologram analysis carried out as described in detail elsewhere (Tomasetti et al, 2004) using the CalcuSyn1 software.
Soft agar colony-forming assay
Cells (104) were seeded in 24-well plates in the RPMI-1640 medium containing 0.35% low melting point (LMP) agar, overlaid with 0.7% LMP agar, and cultured at 37°C in 5% CO2 for 30 days. Every 7 days, 0.5 ml of fresh medium was added to each well. The colonies of cells were treated with α-TOS (30 μM), VK3–AA mixture (3 μM VK3 and 0.4 mM AA), or α-TOS–VK3–AA combination (30 μM α-TOS, 3 μM VK3, and 0.4 mM AA), and after 7 days the colonies were stained with the crystal violet dye and visualised by optical microscope.
Actin–phalloidine labelling
PC3 cells were placed overnight in 35-mm dishes on glass coverslips. After 6 h of incubation with α-TOS or combinations of the agents (VK3-AA and α-TOS–VK3–AA), the cells were washed with PBS, fixed with 4% formaldehyde in PBS, and incubated in the saponin solution (0.05% saponine and 2% FBS in PBS). The cells were then incubated with TRIC-conjugated phalloidine (Sigma) (2 μg ml−1) at room temperature for 30 min, and the coverslips mounted on microscope slides with VectraShield plus DAPI (Vector Laboratories, Burlingame, CA, USA) and inspected in a fluorescence microscope (Zeiss, Axiocam MRc5, Thomwood, NY, USA, magnification × 60).
DNA damage
Damage of DNA was assessed using the alkaline comet assay (Tomasetti et al, 2001). PC3, cells were placed in 96-well flat-bottom tissue culture plate at 104 per well. After overnight incubation, cells were treated with α-TOS (30 μM), VK3–AA mixture (3 μM VK3 and 0.4 mM AA), α-TOS–VK3–AA combination for increasing time periods, and DNA damage was evaluated. To do so, in brief, cells were sandwiched between thin layers of agarose on a microscope slide, lysed at alkaline pH, electrophoresed, stained with the DAPI dye, and inspected in the fluorescence microscope. The number of strand breaks was scored visually such that 100 randomly selected comets were graded according to the degree of damage into five classes (0–4) to provide an overall score for each gel of 0–400 arbitrary units (AU).
Oxygen consumption assay
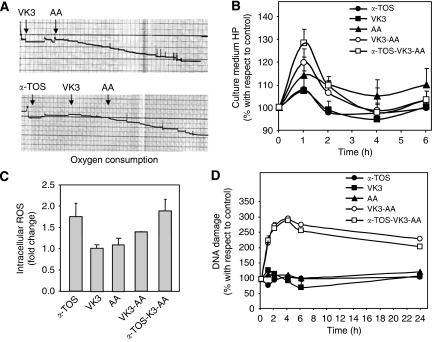
The capacity of α-TOS, VK3, and AA alone or in combination to induce reactive oxygen species (ROS) formation was estimated by evaluating the oxygen consumption using the Clark's oxygen electrode. α-TOS (0.3 mM), VK3 (30 μM), and AA (4 mM) were sequentially added into the Clark's oxygen electrode chamber and oxygen consumption assessed.
Assessment of hydroperoxide formation
Hydroperoxide levels were evaluated in the conditioned medium using the d-ROMs assay (Vassalle et al, 2006). PC3 cells were placed in 96-well flat-bottom tissue culture plates at 104 per well. After overnight incubation, cells were treated with α-TOS (30 μM), VK3 (3 μM) AA (0.4 μM) VK3–AA mixture (3 μM VK3 and 0.4 mM AA), or the α-TOS–VK3–AA combination, and aliquots were taken at different time points. Briefly, 3 μl of medium was added to the reaction mixture containing N,N-diethyl-para-phenylendiamine and acetate buffer (pH 4.8). Samples were subjected to 20 repeated spectrophotometric readings (520 nm). The concentration was automatically calculated from the mean slope (the rate of change in absorbance).
Assessment of generation of intracellular ROS
Intracellular ROS levels were estimated using the fluorescent dye 2′7′-dichlorofluorescein diacetate (DCFA). PC3 cells were seeded in 24-well flat-bottom plates and 20 μM of DCFA, a cell-permeable, ROS-sensitive dye added to each well. After 30 min of incubation, the florescent probe was removed and the cells exposed to α-TOS (30 μM) VK3 (3 μM) AA (0.4 μM) and vitamin combination (VK3-AA and α-TOS–VK3–AA). After a 24-h incubation, the cells were collected, washed, and resuspended in PBS, and analysed by flow cytometry (FACS Calibur, Becton Dickinson, Palo Alto, CA, USA). The level of ROS was detected as fluorescence intensity and expressed as fold change with respect to the control.
Annexin V–propidium ioide (PI) staining
Apoptosis was quantified using the annexin V-FITC method, which detects phosphatidyl serine (PS) externalised in the early phases of apoptosis (Boersma et al, 1996). Cells were plated at 105 per well in 24-well plates. After an overnight incubation, cells were treated with α-TOS (30 μM), VK3–AA mixture (3 μM VK3 and 0.4 mM AA), and α-TOS–VK3–AA combination. Floating and attached cells were collected, washed with PSBS, resuspended in 100 μl binding buffer, incubated for 20 min at room temperature with 2 μl annexin V-FITC, supplemented with 10 μl PI (10 μg ml−1), and analysed by flow cytometry using channel 1 for annexin V-FITC binding and channel 2 for PI staining.
Western blot analysis
PC3 cells were treated with α-TOS (30 μM), VK3–AA mixture (3 μM VK3 and 0.4 mM AA), α-TOS–VK3–AA combination for 24 h. Floating and attached cells were collected, lysed in a buffer containing 250 mM NaCl, 25 mM Tris–HCl (pH 7.5), 5 mM EDTA, 1% Nonidet P-40, and a cocktail of protease inhibitors (2 μg ml−1 aprotinin, 2 μg ml−1 leupeptin, 1 mM phenylmethyl-sulfonyl fluoride, and 2 μg ml−1 proteinin), and stored at −80°C until used. The protein levels were quantified using the Bradford assay (Sigma). The protein samples (50 μg per lane) were boiled for 5 min, resolved using 12.5% SDS–PAGE, and transferred to a nitrocellulose membrane. The membrane was blocked (PBS containing 0.1% Tween and 5% skimmed milk) for 1 h, and incubated overnight with anti-caspase-8, anti-caspase-9, anti-caspase-3, or anti-Bid IgG (all Cell Signaling Technology, Danvers, MA, USA). After incubation with an HRP-conjugated secondary IgG (Sigma), the blots were developed using the ECL detection system (Pierce Biotechnology, Rockford, IL, USA). Band intensities were visualised by ChemiDoc using the Quantity One software (BioRad Laboratories, Hercules, CA, USA). β-Actin was used as a control for protein loading.
Assessment of lysosomal and mitochondrial destabilisation, and inhibition of lysosomes
The integrity of lysosomes and mitochondria was monitored based on the uptake of Acridine orange (AO; Sigma) (Hopkins, 2008) and MitoTracker Red-580 (Molecular Probes, Carlsbad, CA, USA), respectively.
Fluorescent microscopy
PC3 cells were placed in 6-well plates at 3 × 105 per well on glass coverslip. The cells were allowed to attach overnight and then incubated 24 h with α-TOS (30 μM), or vitamin combination (VK3–AA and α-TOS–VK3–AA). After treatment, cells were resuspended in 2 ml RPMI-1640 medium with 5 μg ml−1 AO or 100 nM MitoTracker Red-580, incubated at 37°C for 15 min, mounted on slides with Vectashield (Vector Laboratories) and viewed in a fluorescence microscope (Zeiss, Axiocam MRc5, magnification × 60).
Cytofluorimetry
PC3 cells were plated overnight in a 6-well plate at 3 × 105 per well. After 24 h of treatment with α-TOS (30 μM) or vitamin combination (VK3–AA and α-TOS–VK3–AA), the cells were incubated with 5 μg ml−1 AO for 15 min. Floating and attached cells were collected, resuspended in PBS, and red fluorescence evaluated by flow cytometry. The percentage of cells with low intensity of red fluorescence (pale cells) was used as a marker for the extent of lysosomal destabilisation (impairment of AO uptake).
Lysosmal inhibition
Cells were incubated overnight in the presence of 20 μM E-64d, a broad-spectrum cathepsin and calpain inhibitor, treated with α-TOS or vitamin combination (VK3-AA and α-TOS-VK3- AA) for 24 h, and assessed for viability, mitochondrial and lysosomel integrity, and cytochrome c release.
Cytochrome c release
PC3 cells (3 × 105 per well in 6-well plates) were treated with α-TOS or vitamin combination (VK3–AA and α-TOS–VK3–AA) for 24 h. Cells were then harvested and the pellet resuspended in the digitonin cell permebilization buffer. After incubation on ice for 5 min, the cells were centrifuged at 1000 g for 5 min at 4°C. The supernatant containing the cytosolic fraction of cytochrome c was collected. The remaining pellet was resuspended in the RIPA cell lysis buffer, vortexed, and incubated on ice for 30 min. The lysate was then centrifuged at 10 000 g for 10 min at 4°C. The cytosolic and mitochondrial fractions were then assessed using the enzyme immunometric assay kit (Assay Designs, Hines Drive Ann Arbor, MI, USA). The results were quantified as pg ml−1 and expressed as percentage of cytochrome c in each fraction respect to the total.
Statistical analysis
Data are presented as mean±s.d. Comparisons between groups were carried out using the Mann–Whitney U-test for unpaired samples and Kruskall–Wallis analysis for multiple comparisons. Statistical calculations were carried out using the SPSS statistical package version 12.0F. Statistical differences of at least P<0.05 were considered statistically significant.
Results
αTOS, VK3, and AA exert different toxicity towards prostate cancer cells and fibroblasts
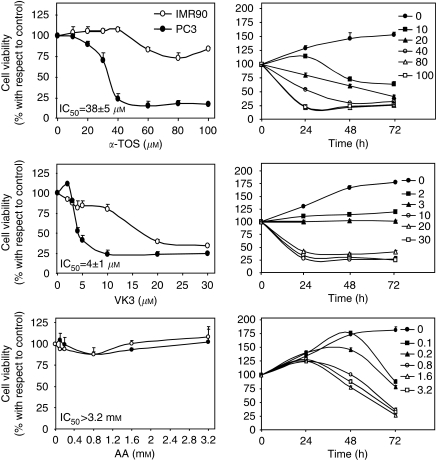
Treatment of PC3 cells with α-TOS and VK3 resulted in dose-dependent cytotoxicity, whereas the cells were completely resistant to AA. Figure 1 (left panel) shows the viability curves of PC3 cells with IC50 value ranging from 30 to 40 μM for α-TOS and 4–5 μM for VK3. The cells were completely resistant to AA treatment up to 3.2 mM, exerting its cytotoxic effect at prolonged exposure times (Figure 1, right panel). The dose- and time-dependent plots show that low doses of α-TOS and AA were not cytotoxic within the initial 24 h of drug exposure, whereas cell death was observed at prolonged time points. No effect of VK3 below 3 μM occurred. It should be noted that non-malignant cells such as fibroblasts were resistant to α-TOS and AA at concentrations up to 100 μM and 3.2 mM, respectively. VK3 was found toxic for the IMR90 fibroblasts at concentrations over 10 μM.
Figure 1.
Cytotoxic effect of α-tocopheryl succinate (α-TOS), vitamin K3 (VK3), and ascorbic acid (AA) on prostate cancer cells and fibroblasts. PC3 and IMR90 cells were seeded into 96-well tissue culture plates at 104 per well and treated with increasing concentration of α-TOS, VK3, and AA. The cells were assessed for viability using the MTT assay following exposure to the agents for 24 h at different concentrations (left panels) or for different times at the concentrations shown (μM) (right panels). The IC50 values were derived from the data in the left panels. All data are expressed as mean±s.d. of the percentage variation with respect to the control (untreated cells) of three independent experiments carried out in duplicate.
Effect of the combination of α-TOS, VK3, and AA on prostate cancer cells
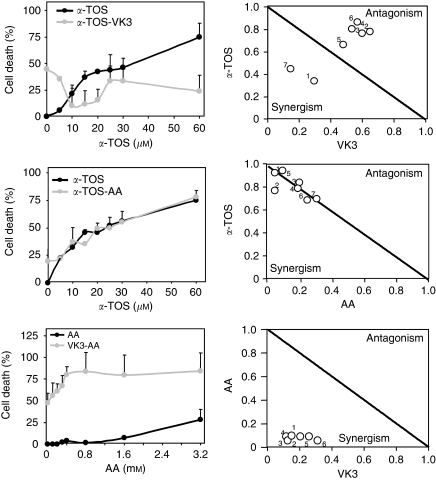
To study the combined effects of α-TOS, VK3, and AA, PC3 cells were exposed to increasing concentrations of individual drugs alone or in combination, and cell death was assessed. The data were then used to carry out isobologram analysis. The IC50 values for one drug <1 were plotted against corresponding IC50 values for the other drug. Distribution of individual points along the diagonal connecting the values of 1 suggests an additive effect of the two drugs, whereas the points below or above the line indicate their synergism and antagonism, respectively (Figure 2, right panel).
Figure 2.
Induction of cell death in prostate cancer cells exposed to α-tocopheryl succinate (α-TOS) in combination with vitamin K3 (VK3) and ascorbic acid (AA). The effect of α-TOS, VK3, and AA on prostate cancer cells was evaluated on the basis of cell death induced by the drugs alone or in combination (left panels). Synergistic, additive or antagonistic effects of sets of two drugs were assessed by constructing the isobolograms at IC50 based on the results of the MTT assay for PC3 cells treated with individual combinations of two drugs. Each isobologram combines the results of at least three independent experiments carried out in duplicate.
Antagonistic effects on cell death induction were found for the combination of α-TOS and VK3, and the presence of α-TOS markedly reduced VK3-induced cell death (Figure 2, left panel). No cytotoxic interaction was observed for the α-TOS–AA combination, whereas a synergistic effect was found for the VK3–AA combination. The cytotoxic effect of VK3 was synergistically enhanced by the addition of increasing doses of AA (Figure 2).
αTOS and VK3–AA in combination exert selective cooperative effect in prostate cancer cells
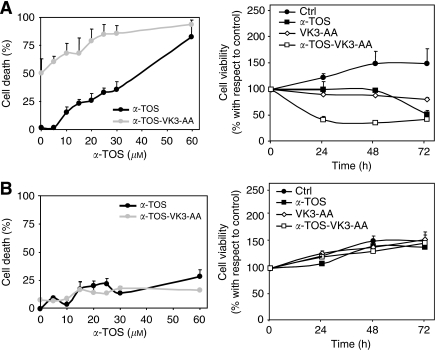
The effect of α-TOS on cytotoxicity induced by the combination of VK3 and AA was evaluated using the PC3 prostate cancer cells and the IMR90 fibroblasts. Cell death induced by the VK3–AA combination was found to be enhanced by α-TOS, and the effect was synergistic/additive (Figure 3A). Combining sub-lethal dose of α-TOS with a VK3 plus AA that alone does not induce cell death caused induction of cell death in PC3 cells after 24 h of exposure to the agents. This effect was selective for the cancer cells, as no cell death was observed when the combination of the three compounds was used to challenge the non-malignant fibroblasts (Figure 3B).
Figure 3.
Effect of α-tocopheryl succinate (α-TOS) alone or in combination with the vitamin K3 (VK3) and ascorbic acid (AA) on cell-death induction and viability of prostate cancer cells and fibroblasts. (A) PC3 and (B) IMR90 cells were exposed to a sub-apoptotic dose of α-TOS (30 μM) alone or in combination with sub-apoptotic levels of VK3 (3 μM) plus AA (0.4 mM). The cells were then assessed for cell death and viability. The results are expressed as mean±s.d. of percentage and of the percentage variation with respect to the control (untreated cells) of three independent experiments carried out in duplicate.
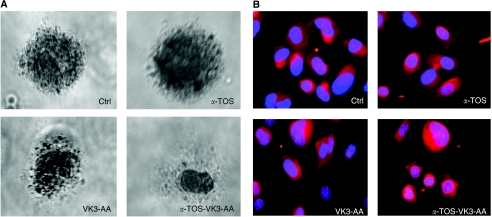
We then verified the morphological changes of PC3 cells treated with the agents alone or in combination. The efficacy of α-TOS to induce cell damage in combination with VK3 plus AA was first evaluated in vitro using the soft-agar colony-forming assay. As shown in Figure 4A, PC3 cells gave rise to numerous and large colonies in soft agar. The colonies were then treated with the drugs alone or in combination. Translucent and pycnotic cells were observed after 1 week of incubation when the agents were added together.
Figure 4.
Morphological changes of prostate cancer cells exposed to α-tocopheryl succinate (α-TOS) and vitamin K3 (VK3) plus ascorbic acid (AA) alone or in combination. (A) For the soft-agar colony-forming assay, cells were seeded in 24-well culture plates at 104 per well in the RPMI-1640 medium containing 0.35% low melting point (LMP) agarose overlaid with 0.7% LMP agarose. The cells were maintained at 37°C in 5% CO2 for 30 days; every 7 days, 500 μl of fresh medium was added to each well. The formed colonies were treated with a sub-lethal dose of α-TOS (30 μM) or VK3 (3 μM) plus AA (0.4 mM), or the three drugs at these doses together, and the cells were stained with crystal violet after 7 days and inspected by optical microscope. (B) PC3 cells were placed overnight in 35-mm dishes on glass coverslips. After a 6-h incubation with α-TOS (30 μM) or VK3 (3 μM) plus AA (0.4 mM), or the three drugs at these concentrations together, the cells were stained with phalloidine–TRIC, and the coverslips mounted on microscope slides with Vectrashield containing DAPI and inspected in a fluorescence microscope.
Phalloidin staining for actin was used to assess the effect of treatment with α-TOS, VK3–AA, and α-TOS in combination with VK3 plus AA on morphology of the cells. After 6-h exposure, cells treated with the combination of the three agents underwent morphological alterations such as formation of cytoplasmic blebs (Figure 4B). These blebs are associated with the preservation and diminution of cell size and suggest that those cells loose pieces through a mechanism of self-excision. Further, the treated cells display a pleiomorphism that makes them elongated, enormous, or smaller than the control tumour cells. The nuclei of the treated cells are not showing condensations typical of apoptotic stages and are enlarged. They also appear with an enhanced inner envelope with excessive phalloidin contrast and show enlarged nucleoli. Nuclei remain unbroken and are not part of the pieces shed by the cells as it would be in apoptotic bodies. Hence, the cell demise appears to demonstrate the characteristics of autoschizis cell death as previously described (Gilloteaux et al, 1998, 2001, 2005).
α-TOS and VK3 plus AA cause generation of ROS
The ability of α-TOS, VK3, and AA, and their combination to induce ROS formation has been assessed using the Clark's oxygen electrode. The agents were sequentially added into the oxygen electrode chamber and consumption of oxygen evaluated. As shown in Figure 5A, VK3 itself did not induce oxygen consumption, which was observed to occur following the addition of AA. Similarly, no oxygen consumption occurred both in the presence of α-TOS and VK3, whereas it was observed in the presence of AA. We next tested generation of ROS induced by incubation of PC3 cells with sub-apoptotic doses of the three agents alone or in combination. The presence of extracellular ROS has been evaluated by the determination of hydroperoxide levels in the conditioned medium of the treated PC3 cells. The levels of ROS were found to increase after 1 h of incubation with VK3 plus AA in the presence of α-TOS (Figure 5B). It should be noted that α-TOS itself was responsible for the observed induction of intracellular ROS formation, whereas VK3 plus AA did not increase the intracellular ROS production any further (Figure 5C).
Figure 5.
Reactive oxygen species (ROS) generation and DNA fragmentation induced in prostate cancer cells by α-tocopheryl succinate (α-TOS) or vitamin K3 (VK3) plus ascorbic acid (AA) alone or in combination. (A) The capacity of α-TOS or VK3 plus AA alone or in combination to induce ROS formation was evaluated by assessing the oxygen consumption using the Clark's oxygen electrode. (B) PC3 cells were placed in 96-well flat-bottom tissue culture plate at 104 per well. After overnight incubation, cells were treated with α-TOS (30 μM), VK3 (3 μM), and AA (0.4 mM) alone or in combination, and aliquots of the conditions media were taken at regular intervals and evaluated for the level of hydroperoxides using the d-ROMs assay. Data are expressed as of percentage variation with respect to the control. (C) Intracellular ROS were estimated using the fluorescent dye 2′7′-dichlorofluorescein diacetate (DCFA). PC3 cells were seeded in 24-well flat-bottom plates and 20 μM of DCFA added. After 30 min of incubation, the cells were exposed to α-TOS (30 μM), VK3 (3 μM), and AA (0.4 mM) alone or in combination. After 24 h, the cells were evaluated by flow cytometry. The amount of ROS was detected as fluorescence intensity normalised for the blank (samples without DCFA) and expressed as fold change with respect to the control (untreated cells). (D) DNA fragmentation was analysed using the alkaline comet assay. PC3 cells were seeded in 96-well flat-bottom tissue culture plates at 104 per well, treated after overnight incubation with α-TOS (30 μM), VK3 (3 μM), and AA (0.4 mM) alone or in combination, and evaluated for DNA damage at different time points. The level of DNA strand breaks was assessed using the comet assay that is based on visual scoring with the maximum of 400 arbitrary units (see Material and Methods for detail). The data are expressed as the percentage variation with respect to the control (untreated cells).
The efficacy of the three agents to induce DNA damage, which was assessed as single strand break (SSB) formation (Figure 5D), was evaluated. VK3 plus AA induced generation of SSBs, which was observed after a 1-h treatment. SSBs induced by VK3 and AA were not repaired and persisted in the cells for up to 24 h. It is interesting to note that α-TOS did not increase the SSB formation observed when the cells were exposed to VK3 plus AA, neither did it affect the DNA repair.
Combination of αTOS, VK3, and AA induces cell death by lysosomal and mitochondrial destabilisation
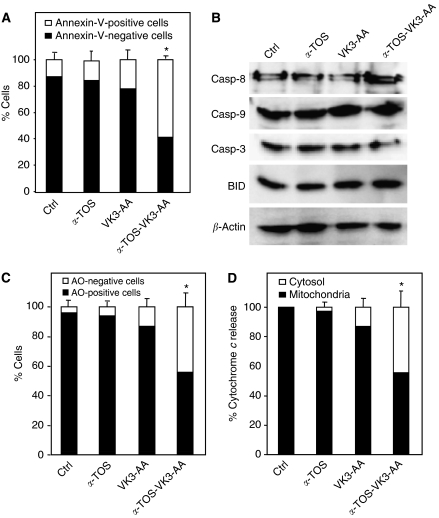
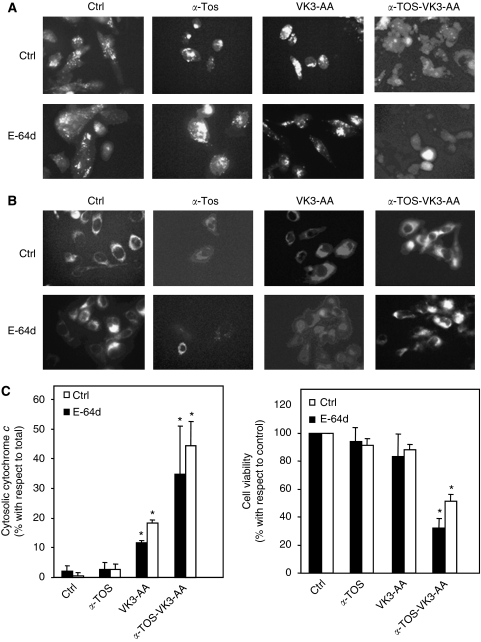
Incubation of PC3 cells with a combination of αTOS, VK3, and AA at a dose at which the individual compounds alone do not induce cell death was found to cause detachment of cells (data not shown) and phosphotidylserine externalisation (Figure 6A). Typically, 50–60% cells were annexin-V positive. However, no PI uptake (data not shown) and no sign of caspase activation (Figure 6B) were observed. We therefore investigated the potential role of the lysosomal/endosomal system, which has a major role in intracellular protein degradation and recycling, as it has been suggested to promote cell death, as shown, for example, for α-TOS (Neuzil et al, 1999, 2002). Therefore, a possible release of lysosomal proteases and mitochondrial cytochrome c to the cytosol have been tested in PC3 cells exposed to α-TOS or VK3 plus AA, as well as to α-TOS with VK3 plus AA. Some 50–60% pale cells (Figure 6C) and cytochrome c release (Figure 6D) were observed in cells treated with the combination of α-TOS, VK3, and AA. Control cells exhibited a punctuated red fluorescence pattern of AO and MitoTracker Red-580, suggesting that the two dyes accumulated in lysosomes and mitochondria, respectively (Figure 7A and B, upper panel). The combination of α-TOS, VK3, and AA caused appearance of cells with substantially decreased red fluorescence due to the loss of lysosomal and mitochondria integrity. Pre-treatment of cells with E-64d reduced neither lysosomal damage nor mitochondrial permeabilisation (Figure 7A and B, lower panel), coinciding with the appearance of cytochrome c in the cytosol (Figure 7C) and cell death induction (Figure 7D).
Figure 6.
Effect of α-tocopheryl succinate (α-TOS) or vitamin K3 (VK3) plus ascorbic acid (AA) alone or in combination on the cell death induction and destabilisation of mitochondria and lysosomes. PC3 cells were exposed for 24 h to sub-apoptotic level of α-TOS (30 μM) or VK3 (3 μM) plus AA (0.4 mM) alone or in combination, and cell death evaluated by the (A) annexin V-propidium ioide staining and (B) caspase activation. Lysosomal destabilisation in cells treated as described above was evaluated by the uptake of (C) Acridine orange (AO), (D) mitochondrial perturbation was assessed by cytochrome c release. The sign ‘*’ denotes significantly different data for treated and control cells with P<0.05.
Figure 7.
Induction of apoptosis by α-tocopheryl succinate (α-TOS) or vitamin K3 (VK3) plus ascorbic acid (AA) alone or in combination in the presence of a lysosomal protease inhibitor. PC3 cells were exposed for 24 h to sub-apoptotic doses of α-TOS (30 μM) or VK3 (3 μM) plus AA (0.4 mM) alone or in combination, and (A) lysosmal and (B) mitochondrial destabilisation were evaluated using the Acridine orange (AO) and MitoTracker Red-580 staining, respectively, in the presence or absence of the lysosomal protease inhibitor E-64d. (C) Cytochrome c release and cell viability after treatments of prostate cancer cells as described above in the presence or absence of lysosomal protease inhibitor E-64d was assessed. The sign ‘*’ denotes significantly different data for treated and control cells with P<0.05.
Discussion
Selective therapy for neoplastic pathologies has not been found thus far, which presents a major obstacle in efficient cancer management (Hopkins, 2008). Cancer treatment requires modulation of concrete targets, which can be compromised by the compensatory mechanisms and/or mutations (Stelling et al, 2004; Kitano, 2007). Overcoming these problems often requires high drug doses that may, on the other hand, promote deleterious effects to non-cancerous tissues (Kassouf et al, 2005). Combinations of two or more agents that exert a synergistic effect can overcome the undesirable toxicity and other side-effects associated with high doses of single drugs, allowing a reduced dosage of each compound.
We therefore tested exposure of prostate cancer cells to three agents, α-TOS, VK3, and AA, widely studied as anti-cancer compounds, alone or in combination (Chen et al, 2005; Ogawa et al, 2007; Neuzil et al, 2007b). α-TOS and VK3 were both highly cytotoxic towards prostate cancer cells, whereas lethal effects of AA were observed only at prolonged times of exposure (c.f. Figure 1). An antagonistic interaction was found for the combination of α-TOS and VK3, whereas AA did not exert any effect when combined with α-TOS. As previously described (De Loecker et al, 1993; Jamison et al, 1996; Verrax et al, 2005; Tareen et al, 2008; Beck et al, 2009) and also observed in this study, an efficient synergistic effect on cell viability was observed for the pro-oxidant mixture containing pharmacological doses of AA and a redox-active compound such as menadione (VK3), (c.f. Figure 2). Indeed, the combination of AA and the redox-cycling quinone VK3 promotes oxidative stress that may kill cancer cells (Taper and Roberfroid, 1992; Taper et al, 2001). Oral administration of the VK3–AA mixture in the ratio 1 : 100 (the Apatone preparation) significantly increased the mean survival time of nude mice inoculated i.p. with the DU145 prostate cancer cells and significantly reduced the growth rate of solid tumours without inducing any significant bone marrow toxicity and pathological changes of non-tumour tissues (Jamison et al, 2005). Further, the safety and efficacy of oral Apatone supplementation were demonstrated in patients with prostate cancer resilient to standard therapy (Tareen et al, 2008). However, the potential long-term effect of Apatone on the disease progression and possible secondary side-effects are not known and remain to be investigated.
To reduce the pharmacological doses of the agents, we combined VK3 plus AA at concentrations that themselves do not induce apoptosis with sub-apoptotic levels of α-TOS. This combination of the three drugs was efficient in induction of cell death in a selective manner that appears to be as autoschizis cell death (c.f. Figures 3 and 4). The soft-agar colony-forming assay was carried out to mimic the in vivo situation, where tumour cells grow as masses. Soft-agar assay and phalloidin staining of actin have been employed to reveal a potential effect of the treatment with α-TOS, VK3, and AA, and with VK3–AA–α-TOS on the morphology of the cells. PC3 cells exposed to the combination of VK3, AA and α-TOS displayed blebs and membrane alterations related to cytoskeleton changes (c.f. Figure 4). The cells also significantly decreased their size and changed their shape similarly to those found after a combined VK3–AA treatment (Gilloteaux et al, 2005). The specificity of the anti-tumour activity of VK3 plus AA is related to their ability to induce ROS formation (Venugopal et al, 1996). We observed oxygen consumption only in the presence of both VK3 and AA. Although the sub-apoptotic dose of the combination of VK3 with AA resulted in the appearance of hydroperoxides in the extracellular compartment, intracellular generation of ROS and persistent DNA damage, the cells died only in the presence of α-TOS (c.f. Figure 5). We observed that cell death in cells treated with the combination of α-TOS, VK3, and AA proceeded without caspases activation (c.f. Figure 6). A caspase-3 independent cell death was previously observed in leukaemia cells after VK3–AA treatment (Verrax et al, 2005). This can be reconciled with the notion that peroxidation of the plasma membrane of cells may favour unregulated increase in intracellular levels of calcium as previously observed (Sakagami and Satoh, 1997), which, along with thiol oxidation, may result in mitochondrial destabilisation.
Mitochondria has recently emerged as the effective target for anti-cancer drugs (Fantin and Leder, 2006; Gogvadze et al, 2008). α-TOS, a compound epitomising the ‘mitocan’ group of anti-cancer agents (Neuzil et al, 2007a), was found to induce cell death by generation of superoxide anion radicals by targeting complex II of the mitochondria respiratory chain (Dong et al, 2008, 2009). ROS, in turn, promote apoptosis by catalysing the formation of disulfite bridges between monomeric Bax, resulting in the formation of mitochondrial outer membrane channel. ROS also cause oxidation of cardiolipin, triggering the release of cytochrome c and its translocation through the Bax channel (d’Alessio et al, 2005; Neuzil et al, 2006). Mitochondrial channel in response to α-TOS can also be formed by transcriptional upregulation of Noxa, which results in formation of Bak oligomeric structures (Neuzil et al, submitted).
In addition, ROS cause destabilisation of lysosomes, presumably leading to cytosolic translocation of various proteases (Neuzil et al, 1999, 2002). Lysosomal destabilisation as well as cytosolic release of cytochrome c were observed in cells exposed to α-TOS in combination with VK3 plus AA. It was shown earlier that perturbation of lysosomes results in cell death, mostly by autoschizis that is dependant on mitochondria (Boya et al, 2003). We observed in this study that the inhibitor of lysosomal destabilisation did not attenuate mitochondrial ‘leakage’, leading to cytochrome c release, and subsequent cell death (c.f. Figures 6 and 7). This reinforces the concept that mitochondrial destabilisation constitutes a central event in the programmed cell death such as apoptosis and autoschizis (Verrax et al, 2005). Again, this evokes the possibility that primary thiol oxidation of mitochondrial proteins, derived from an oxidative shift in the cellular redox potential (Venugopal et al, 1996), can induce mitochondrial membrane permeabilisation (Zamzami et al, 1998). Moreover, oxidation of critical thiols within the catalytic centre of caspases annihilates their latent proteolytic potential and, thus, preclude their auto-activation (Mannick et al, 2001).
We conclude that α-TOS synergistically cooperates with VK3 plus AA in the induction of prostate cancer cell apoptosis. The combination of VK3, AA, and α-TOS induces cell death that is selective for cancer cells and proceeds through a caspase-independent pathway. We propose that addition of α-TOS at sub-apoptotic doses may be considered for cancer therapy when high doses of established drugs are required.
Acknowledgments
This work was supported by grant ‘Finalised Project’ from Italian Ministry of Health.
References
- Beauchamp MC, Knafo A, Yasmeen A, Carboni JM, Gottardis MM, Pollak MN, Gotlieb WH (2009) BMS-536924 sensitizes human epithelial ovarian cancer cells to the PARP inhibitor, 3-aminobenzamide. Gynecol Oncol 155(2): 193–198 [DOI] [PubMed] [Google Scholar]
- Beck R, Verrax J, Dejeans N, Taper H, Calderon PB (2009) Menadione reduction by pharmacological doses of ascorbate induces an oxidative stress that kills breast cancer cells. Int J Toxicol 28: 33–42 [DOI] [PubMed] [Google Scholar]
- Boersma AWM, Nooter K, Oostrum RG, Stoter G (1996) Quantification of apoptotic cells with fluorescein isothiocyanate-labeled annexin V in Chinese Hamster ovary cell cultures treated with cisplatin. Cytometry 24: 123–130 [DOI] [PubMed] [Google Scholar]
- Boya P, Andreau K, Poncet D, Zamzami N, Perfettini JL, Metivier D, Ojcius DM, Jäättelä M, Kroemer G (2003) Lysosomal membrane permeabilization induces cell death in a mitochondrion-dependent fashion. J Exp Med 197: 1323–1934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buc-Calderon P, Praet M, Ruysschaert JM, Roberfroid M (1989) Increasing therapeutic effect and reducing toxicity of doxorubicin by N-acyl dehydroalanines. Eur J Cancer Clin Oncol 25: 679–685 [DOI] [PubMed] [Google Scholar]
- Carmichael J, DeGraff WG, Gazdar AF, Minna JD, Mitchell JB (1987) Evaluation of a tetrazolium-based semiautomated colorimetric assay: assessment of radiosensitivity. Cancer Res 47: 943–946 [PubMed] [Google Scholar]
- Chen Q, Espey MG, Sun AY, Lee JH, Krishna MC, Shacter E, Choyke PL, Pooput C, Kirk KL, Buettner GR, Levine M (2007) Ascorbate in pharmacologic concentrations selectively generates ascorbate radical and hydrogen peroxide in extracellular fluid in vivo. Proc Natl Acad Sci USA 104: 8749–8754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Espey MG, Krishna MC, Mitchell JB, Corpe CP, Buettner GR, Shacter E, Levine M (2005) Pharmacologic ascorbic acid concentrations selectively kill cancer cells: action as a pro-drug to deliver hydrogen peroxide to tissues. Proc Natl Acad Sci USA 102: 13604–13609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Alessio M, De Nicola M, Coppola S, Gualandi G, Pugliese L, Cerella C, Cristofanon S, Civitareale P, Ciriolo MR, Bergamaschi A, Magrini A, Ghibelli L (2005) Oxidative Bax dimerization promotes its translocation to mitochondria independently of apoptosis. FASEB J 19: 1504–1506 [DOI] [PubMed] [Google Scholar]
- De Loecker W, Janssens J, Bonte J, Taper HS (1993) Effects of sodium ascorbate (vitamin C) and 2-methyl-1,4-naphthoquinone (vitamin K3) treatment on human tumor cell growth in vitro. II. Synergism with combined chemotherapy action. Anticancer Res 13: 103–106 [PubMed] [Google Scholar]
- Dong LF, Freeman R, Liu J, Zobalova R, Marin-Hernandez A, Stantic M, Rohlena J, Valis K, Rodriguez-Enriquez S, Butcher B, Goodwin J, Brunk UT, Witting PK, Moreno-Sanchez R, Scheffler IE, Ralph SJ, Neuzil J (2009) Suppression of tumor growth in vivo by the mitocan alpha-tocopheryl succinate requires respiratory complex II. Clin Cancer Res 15: 1593–1600 [DOI] [PubMed] [Google Scholar]
- Dong LF, Low P, Dyason J, Wang XF, Prochazka L, Witting PK, Freeman R, Swettenham E, Valis K, Liu J, Zobalova R, Turanek J, Spitz DR, Domann FE, Scheffler IE, Ralph SJ, Neuzil J (2008) α-Tocopheryl succinate induces apoptosis by targeting ubiquinone-binding sites in mitochondrial respiratory complex II. Oncogene 27: 4324–4335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichhorn ME, Ischenko I, Luedemann S, Strieth S, Papyan A, Werner A, Bohnenkamp H, Guenzi E, Preissler G, Michaelis U, Jauch KW, Bruns CJ, Dellian M (2010) Vascular targeting by EndoTAG-1 enhances therapeutic efficacy of conventional chemotherapy in lung and pancreatic cancer. Int J Cancer 126(5): 1235–1245 [DOI] [PubMed] [Google Scholar]
- Fantin VR, Leder P (2006) Mitochondriotoxic compounds for cancer therapy. Oncogene 25: 4787–4797 [DOI] [PubMed] [Google Scholar]
- Gogvadze V, Orrenius S, Zhivotovsky B (2008) Mitochondria in cancer cells: what is so special about them? Trends Cell Biol 18: 165–173 [DOI] [PubMed] [Google Scholar]
- Gilloteaux J, Jamison JM, Arnold D, Ervin E, Eckroat L, Docherty JJ, Neal D, Summers JL (1998) Cancer cell necrosis by autoschizis: synergism of antitumor activity of vitamin C: vitamin K3 on human bladder carcinoma T24 cells. Scanning 20: 564–575 [DOI] [PubMed] [Google Scholar]
- Gilloteaux J, Jamison JM, Arnold D, Taper HS, Summers JL (2001) Ultrastructural aspects of autoschizis: a new cancer cell death induced by the synergistic action of ascorbate/menadione on human bladder carcinoma cells. Ultrastruct Pathol 25: 183–192 [DOI] [PubMed] [Google Scholar]
- Gilloteaux J, Jamison JM, Neal DR, Summers JL (2005) Cell death by autoschizis in TRAMP prostate carcinoma cells as a result of treatment by ascorbate: menadione combination. Ultrastruct Pathol 29: 221–235 [DOI] [PubMed] [Google Scholar]
- Hopkins AL (2008) Network pharmacology: the next paradigm in drug discovery. Nat Chem Biol 4: 682–690 [DOI] [PubMed] [Google Scholar]
- Jamison JM, Gilloteaux J, Venugopal M, Koch JA, Sowick C, Shah R, Summers JL (1996) Flow cytometric and ultrastructural aspects of the synergistic antitumor activity of vitamin C-vitamin K3 combinations against human prostatic carcinoma cells. Tissue Cell 28: 687–701 [DOI] [PubMed] [Google Scholar]
- Jamison JM, Gilloteaux J, Taper HS, Buc Calderon P, Perlaky L, Thiry M (2005) The in vitro and in vivo antitumor activity of vitamin C:K3 combinations against prostate cancer. In: Trends in Prostate Cancer Research, Lucas JL (ed) 189–236. Nova Science Publishers: Hauppauge, NY [Google Scholar]
- Kassouf W, Dinney CP, Brown G, McConkey DJ, Diehl AJ, Bar-Eli M, Adam L (2005) Uncoupling between epidermal growth factor receptor and downstream signals defines resistance to the antiproliferative effect of Gefitinib in bladder cancer cells. Cancer Res 65: 10524–10535 [DOI] [PubMed] [Google Scholar]
- Keith CT, Borisy AA, Stockwell BR (2005) Multicomponent therapeutics for networked systems. Nat Rev Drug Discov 4: 71–78 [DOI] [PubMed] [Google Scholar]
- Kitano H (2007) Robustness-based approach to systems-oriented drug design. Nat Rev Drug Discov 6: 202–210 [DOI] [PubMed] [Google Scholar]
- Koch N, van Driel IR, Gleeson PA (2000) Hijacking a chaperone: manipulation of the MHC class II presentation pathway. Immunol Today 21: 546–550 [DOI] [PubMed] [Google Scholar]
- Lehár J, Krueger AS, Avery W, Heilbut AM, Johansen LM, Price ER, Rickles RJ, Short III GF, Staunton JE, Jin X, Lee MS, Zimmermann GR, Borisy AA (2009) Synergistic drug combinations tend to improve therapeutically relevant selectivity. Nat Biotechnol 27: 659–666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu W, Dong Z, Donawho C, Fidler IJ (2002) Specific immunotherapy against occult cancer metastases. Int J Cancer 100: 480–485 [DOI] [PubMed] [Google Scholar]
- Mannick JB, Schonhoff C, Papeta N, Ghafourifar P, Szibor M, Fang K, Gaston B (2001) S-nitrosylation of mitochondrial caspases. J Cell Biol 154: 1111–1116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuzil J, Dong LF, Ramanathapuram L, Hahn T, Chladova M, Wang XF, Zobalova R, Prochazka L, Gold M, Freeman RE, Turanek J, Akporiaye ET, Dyason J, Ralph SJ (2007b) Vitamin E analogues: a novel group of mitocans, anti-cancer agents that act by targeting mitochondria. Mol Aspects Med 28: 607–645 [DOI] [PubMed] [Google Scholar]
- Neuzil J, Svensson I, Weber T, Weber C, Brunk UT (1999) α-Tocopheryl succinate-induced apoptosis in Jurkat T cells involves caspase-3 activation, and both lysosomal and mitochondrial destabilisation. FEBS Lett 445: 295–300 [DOI] [PubMed] [Google Scholar]
- Neuzil J, Tomasetti M, Mellick AS, Alleva R, Salvatore BA, Birringer M, Fariss MW (2004) Vitamin E analogues: a new class of inducers of apoptosis with selective anti-cancer effects. Curr Cancer Drug Targets 4: 355–372 [DOI] [PubMed] [Google Scholar]
- Neuzil J, Tomasetti M, Zhao Y, Dong LF, Birringer M, Wang XF, Low P, Wu K, Salvatore BA, Ralph SJ (2007a) Vitamin E analogs, a novel group of ‘mitocans’ as anticancer agents: the importance of being redox-silent. Mol Pharmacol 71: 1185–1199 [DOI] [PubMed] [Google Scholar]
- Neuzil J, Wang XF, Dong LF, Low P, Ralph SJ (2006) Molecular mechanism of ′mitocan′-induced apoptosis in cancer cells epitomizes the multiple roles of reactive oxygen species and Bcl-2 family proteins. FEBS Lett 580: 5125–5129 [DOI] [PubMed] [Google Scholar]
- Neuzil J, Weber T, Gellert N, Weber C (2001a) Selective cancer cell killing by α-tocopheryl succinate. Br J Cancer 84: 87–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuzil J, Weber T, Schröder A, Lu M, Ostermann G, Gellert N, Mayne GC, Olejnicka B, Nègre-Salvayre A, Sticha M, Coffey RJ, Weber C (2001b) Induction of apoptosis in cancer cells by α-tocopheryl succinate: Molecular pathways and structural requirements. FASEB J 15: 403–415 [DOI] [PubMed] [Google Scholar]
- Neuzil J, Zhao M, Ostermann G, Sticha M, Gellert N, Weber C, Eaton JW, Brunk UT (2002) α-Tocopheryl succinate, an agent with in vivo anti-tumour activity, induces apoptosis by causing lysosomal instability. Biochem J 362: 709–715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa M, Nakai S, Deguchi A, Nonomura T, Masaki T, Uchida N, Yoshiji H, Kuriyama S (2007) Vitamins K2, K3 and K5 exert antitumor effects on established colorectal cancer in mice by inducing apoptotic death of tumor cells. Int J Oncol 31: 323–331 [PubMed] [Google Scholar]
- Rhee SG (2006) Cell signaling. H2O2, a necessary evil for cell signaling. Science 312: 1882–1883 [DOI] [PubMed] [Google Scholar]
- Roberts WK, Livingston PO, Agus DB, Pinilla-Ibarz J, Zelenetz A, Scheinberg DA (2002) Vaccination with CD20 peptides induces a biologically active, specific immune response in mice. Blood 99: 3748–3755 [DOI] [PubMed] [Google Scholar]
- Sakagami H, Satoh K (1997) Modulating factors of radical intensity and cytotoxic activity of ascorbate. Anticancer Res 17: 3513–3520 [PubMed] [Google Scholar]
- Stapelberg M, Gellert N, Swettenham E, Tomasetti M, Witting PK, Procopio A, Neuzil J (2005) α-Tocopheryl succinate inhibits malignant mesothelioma by disrupting the FGF autocrine loop: the role of oxidative stress. J Biol Chem 280: 25369–25376 [DOI] [PubMed] [Google Scholar]
- Stelling J, Sauer U, Szallasi Z, Doyle III FJ, Doyle J (2004) Robustness of cellular functions. Cell 118: 675–685 [DOI] [PubMed] [Google Scholar]
- Taper HS, Roberfroid M (1992) Non-toxic sensitization of cancer chemotherapy by combined vitamin C and K3 pretreatment in a mouse tumor resistant to oncovin. Anticancer Res 12: 1651–1654 [PubMed] [Google Scholar]
- Taper HS, Jamison JM, Gilloteaux J, Gwin CA, Gordon T, Summers JL (2001) In vivo reactivation of DNases in implanted human prostate tumors after administration of a vitamin C/K(3) combination. J Histochem Cytochem 49: 109–120 [DOI] [PubMed] [Google Scholar]
- Tareen B, Summers JL, Jamison JM, Neal DR, McGuire K, Gerson L, Diokno A (2008) A 12 week, open label, phase I/IIa study using apatone for the treatment of prostate cancer patients who have failed standard therapy. Int J Med Sci 5: 62–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasetti M, Alleva R, Borghi B, Collins AR (2001) In vivo supplementation with coenzyme Q10 enhances the recovery of human lymphocytes from oxidative DNA damage. FASEB J 15: 1425–1427 [DOI] [PubMed] [Google Scholar]
- Tomasetti M, Rippo MR, Alleva R, Moretti S, Andera L, Neuzil J, Procopio A (2004) α-Tocopheryl succinate and TRAIL selectively synergise in apoptosis induction in human malignant mesothelioma cells. Br J Cancer 90: 1644–1653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trachootham D, Alexandre J, Huang P (2009) Targeting cancer cells by ROS-mediated mechanisms: a radical therapeutic approach? Nat Rev Drug Discov 8: 579–591 [DOI] [PubMed] [Google Scholar]
- Uwagawa T, Chiao PJ, Gocho T, Hirohara S, Misawa T, Yanaga K (2009) Combination chemotherapy of nafamostat mesilate with gemcitabine for pancreatic cancer targeting NF-κB activation. Anticancer Res 29: 3173–3178 [PubMed] [Google Scholar]
- Vassalle C, Boni C, Di Cecco P, Ndreu R, Zucchelli GC (2006) Automation and validation of a fast method for the assessment of in vivo oxidative stress levels. Clin Chem Lab Med 44: 1372–1375 [DOI] [PubMed] [Google Scholar]
- Venugopal M, Jamison JM, Gilloteaux J, Koch JA, Summers M, Giammar D, Sowick C, Summers JL (1996) Synergistic antitumor activity of vitamins C and K3 on human urologic tumor cell lines. Life Sci 59: 1389–1400 [DOI] [PubMed] [Google Scholar]
- Verrax J, Delvaux M, Beghein N, Taper H, Gallez B, Buc Calderon P (2005) Enhancement of quinone redox cycling by ascorbate induces a caspase-3 independent cell death in human leukaemia cells. An in vitro comparative study. Free Radic Res 39: 649–657 [DOI] [PubMed] [Google Scholar]
- Wang XF, Birringer M, Dong LF, Veprek P, Low P, Swettenham E, Stantic M, Yuan LH, Zobalova R, Wu K, Ralph SJ, Ledvina M, Neuzil J (2007) A peptide adduct of vitamin E succinate targets breast cancer cells with high erbB2 expression. Cancer Res 67: 3337–3344 [DOI] [PubMed] [Google Scholar]
- Zahorowska B, Crowe PJ, Yang JL (2009) Combined therapies for cancer: a review of EGFR-targeted monotherapy and combination treatment with other drugs. J Cancer Res Clin Oncol 135: 1137–1148 [DOI] [PubMed] [Google Scholar]
- Zamzami N, Marzo I, Susin SA, Brenner C, Larochette N, Marchetti P, Reed J, Kofler R, Kroemer G (1998) The thiol crosslinking agent diamide overcomes the apoptosis-inhibitory effect of Bcl-2 by enforcing mitochondrial permeability transition. Oncogene 16: 1055–1063 [DOI] [PubMed] [Google Scholar]
- Zhu Z, Haegele AD, Thompson HJ (1997) Effect of caloric restriction on pre-malignant and malignant stages of mammary carcinogenesis. Carcinogenesis 18: 1007–1012 [DOI] [PubMed] [Google Scholar]