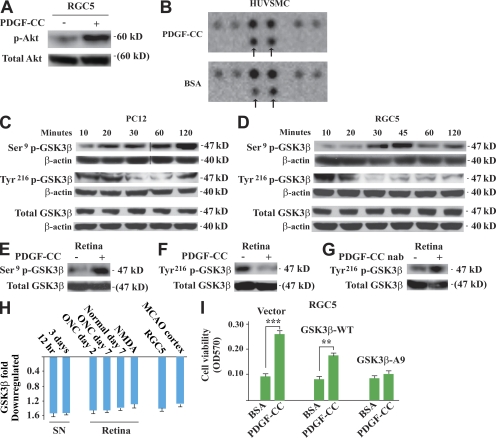
Figure 7.
Neuroprotective effect of PDGF-CC is achieved by regulating GSK3β phosphorylation. (A) PDGF-CC protein treatment activated Akt significantly in cultured RGC5 cells. (B) In a phospho-MAPK array screening assay, PDGF-CC treatment increased GSK3β Ser9 phosphorylation specifically (top, arrows) in HUVSMCs, whereas the phosphorylation of the other molecules remained unchanged. (C and D) In cultured PC12 (C) and RGC5 (D) neuronal cells, PDGF-CC protein treatment increased GSK3β Ser9 phosphorylation and decreased GSK3β Tyr216 phosphorylation, respectively, in a time-dependent manner. Black lines indicate that intervening lanes have been spliced out. (E and F) PDGF-CC protein treatment increased GSK3β Ser9 phosphorylation (E) and decreased GSK3β Tyr216 phosphorylation (F), respectively, in the retina in vivo. (G) PDGF-CC neutralizing antibody treatment increased GSK3β Tyr216 phosphorylation in the retina in vivo. (H) PDGF-CC protein treatment inhibited GSK3β expression in different types of neuronal tissues/cells, including SN, retina (normal or with ONC/NMDA injury), RGC5 cells, and brain cortex with MCAO as measured by real-time PCR. (I) PDGF-CC protein treatment protected RGC5 cells from H2O2-induced cell death in the control cells and cells expressing wild-type GSK3β (GSK3β-WT). The protective effect of PDGF-CC diminished in the RGC5 cells expressing mutant GSK3β, in which Ser9 was mutated to alanine (GSK3β-A9). **, P < 0.01; ***, P < 0.001. The data are represented as means ± SEM of the number of determinations. Experiments, except B, were repeated independently twice with similar results. Representative images (A and C–G) and experiments are shown. nab, neutralizing antibody.