Abstract
AIM
Aclidinium bromide is a muscarinic antagonist in development for the treatment of chronic obstructive pulmonary disease (COPD). This phase I trial in healthy subjects investigated the bronchodilator activity of aclidinium and its ability to reduce methacholine-induced bronchoconstriction.
METHODS
This double-blind, partial-crossover study randomized 12 subjects to treatment with single doses of aclidinium (50, 300 or 600 µg) or placebo. Drug activity was assessed for 24 h after administration by specific airway conductance (sGaw), airways resistance (Raw) and bronchial responsiveness (PC35 sGaw methacholine).
RESULTS
Aclidinium significantly increased sGaw compared with placebo at all assessments and doses (sGaw mean ± SD AUC (l kPa−1 h) for placebo 24.4 ± 4.37, for 50 µg 29.0 ± 7.08, for 300 µg 31.2 ± 6.68 and for 600 µg 32.7 ± 7.95) (P < 0.009), except 50 µg at 1 and 24 h. Significant decreases in Raw were observed with aclidinium 300 and 600 µg compared with placebo at all assessments (Raw mean ± SD AUC (kPa s−1 l−1 h) for placebo 7.7 ± 3.46, for 300 µg 5.8 ± 2.33, for 600 µg 6.3 ± 3.11) (P < 0.04) except 600 µg at 24 h. Differences between aclidinium 300 and 600 µg vs. placebo in PC35 doubling concentration were significant at all assessments (mean ± SD AUC (mg ml−1 h) for placebo 100.0 ± 30.27, for 50 µg 117.2 ± 33.33, for 300 µg 168.9 ± 28.66 and for 600 µg 179.1 ± 15.73 (P < 0.0001). For all endpoints, there was a significant difference between aclidinium 50 µg and the higher doses (P < 0.0001). Aclidinium was not detected in plasma and was well tolerated.
CONCLUSION
Aclidinium produced statistically significant and sustained bronchodilation over 24 h, suggesting long-acting efficacy and providing a rationale for future studies in patients with COPD.
Keywords: aclidinium, anticholinergic, bronchodilator, COPD, long-acting, phase I
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
Long-acting inhaled bronchodilators are central in the symptomatic treatment of patients with chronic obstructive pulmonary disease (COPD). Until now the only long-acting inhaled anticholinergic drug available in the clinic is tiotropium bromide.
WHAT THIS STUDY ADDS
Aclidinium bromide is a new anticholinergic drug. In normal volunteers inhalation of this compound induced a long-lasting bronchodilation and offered protection against the bronchoconstrictor effect of inhaled methacholine. Aclidinium bromide is now being developed as a long-acting anticholinergic for patients with COPD.
Introduction
Chronic obstructive pulmonary disease (COPD) is a preventable and treatable lung disease characterized by chronic airflow limitation that is not fully reversible [1]. The chronic airflow limitation observed in COPD is caused by chronic lung inflammation and damage to airway smooth muscles, often resulting from persistent smoking [2, 3].
Airway smooth muscles are controlled by autonomic nerves, with the cholinergic parasympathetic nervous system regulating airway tone, smooth muscle contraction, vasodilatation and submucosal gland secretion [4]. This system acts as the dominant bronchoconstrictor neural pathway through activation of the vagus nerve. Cholinergic nerves exert their effect through acetylcholine, activating muscarinic receptors [4–6].
Cholinergic tone is the major reversible component of COPD, and may be increased in patients with this condition compared with healthy individuals [7, 8]. Thus, anticholinergic therapy has a clear role in the treatment of COPD, and bronchodilation with anticholinergic agents is an important part of the management of this disease [1]. The use of anticholinergics in the treatment of COPD has been demonstrated with ipratropium bromide. However, the bronchodilatory effect of ipratropium bromide is short-acting and it must be taken every 6–8 h [9]. Currently, the only available long-acting anticholinergic drug is tiotropium bromide [10, 11]. Given the high prevalence of COPD, the significant morbidity and mortality with which it is associated, the variability in individual patient responses and the paucity of effective agents [12, 13], development of additional treatment options is clearly warranted.
Aclidinium bromide is a new long-acting muscarinic antagonist currently in phase III clinical development for the treatment of COPD. Preclinical studies have shown that aclidinium has a long residence time at the M3 receptor and a shorter residence time at the M2 receptor [14]. Aclidinium is also rapidly hydrolyzed (t1/2= 2.4 min) in human plasma to two major inactive metabolites [14]. These pharmacological properties give aclidinium a unique profile amongst the anticholinergic medications which could offer significant advantages to the COPD patient.
The long-lasting activity of aclidinium at M3 receptors, its fast onset of action [15] and its rapid plasma clearance suggest that this new agent will offer sustained bronchodilation with a reduced potential for systemic side-effects. This phase I trial was designed to examine the bronchodilator activity of aclidinium and its ability to reduce methacholine-induced bronchoconstriction in healthy subjects as an anticholinergic model. The pharmacokinetics, safety and tolerability of aclidinium were also investigated.
Methods
Study subjects
Healthy male, non-smoking volunteers, aged 18–45 years, were eligible for participation. At screening, subjects had to demonstrate responsiveness to methacholine challenge (minimum 35% decrease [PC35] in specific airway conductance [sGaw] with a methacholine concentration <32 mg ml−1, starting from 1 mg ml−1), have a body mass index (BMI) between 17 and 29.9 kg m−2, normal vital signs (blood pressure, heart rate and body temperature) and no electrocardiogram (ECG) or routine laboratory assessment abnormalities. Subjects were excluded if they had any relevant medical history including asthma, history of a severe allergic reaction, drug or alcohol abuse, or excessive (>6 glasses or cups a day) intake of xanthine-containing drinks (coffee, tea, chocolate or cola), or who had suffered from a common cold or viral infection of the airways at any time in the 4-week period before screening. Subjects who discontinued the study were replaced, except when withdrawal was due to a drug-related adverse event (AE). All subjects gave written, informed consent and the study was approved by the local ethics committee.
Baseline characteristics of the 12 subjects randomized into the study are shown in Table 1. There were no clinically relevant findings from the medical history or physical examination. One subject withdrew because of intercurrent viral meningitis before receiving the third dose of study drug (aclidinium 600 µg).
Table 1.
Demographic and baseline characteristics (n= 12)
| Mean (SD) | 95% CI | |
|---|---|---|
| Age (years) | 31.5 (8.00) | 26.4, 36.6 |
| BMI (kg m−2) | 24.0 (3.38) | 21.9, 26.2 |
| sGaw (l kPa−1 s−1) | 1.18 (0.39) | 0.93, 1.43 |
| Raw (kPa s−1 l−1) | 0.26 (0.11) | 0.19, 0.33 |
| PC35 sGaw methacholine (mg ml−1) | 15.00 (7.78) | 10.1, 19.9 |
BMI, body mass index; sGaw, specific airway conductance; Raw, airways resistance; PC35 sGaw methacholine, concentration of methacholine causing a 35% decrease in sGaw from baseline.
Study design
This was a single-site, double-blind, randomized, placebo-controlled, incomplete-crossover, phase I trial. Following the screening visit, subjects were randomized to one of four treatment sequences (three subjects per sequence), with each sequence comprising three study visits during which a single dose of randomized medication was administered. There was a washout period of at least 6 days between each treatment. Aclidinium was supplied by Almirall, Barcelona, Spain and administered by a dry powder inhaler (Cyclohaler®, Pharmachemie, Haarlem, the Netherlands) under fasting conditions. Subjects were admitted to the hospital the evening before treatment and remained for 24 h after drug administration.
A low dose of 50 µg was selected as the starting dose. Preclinical studies have shown that the no-observed-adverse-effect-level of aclidinium administered via inhalation is approximately 2500 times this starting dose [16, 17]. Escalation to higher doses of aclidinium (i.e. 300 µg and 600 µg) was only performed when the safety of the 50 µg dose had been fully evaluated.
Excessive physical exercise, consumption of alcohol, grapefruits (including juice), quinine-containing products and xanthine-containing food or beverages were forbidden during the study. No concomitant drugs were allowed, including over-the-counter medication, except paracetamol.
Assessments
Lung function
Airway resistance (Raw) and functional residual capacity (FRC) were measured by standard methodology [18, 19], using a variable-pressure, constant-volume, whole-body plethysmograph (Jaeger, Germany). Plethysmography was performed at screening, before drug administration (baseline) and at 1, 2, 4, 6, 8, 12 and 24 h post-administration with the subject in the seated position. Raw measured at FRC was converted to airway conductance (Gaw) and expressed as specific conductance (sGaw = Gaw/FRC). Five technically satisfactory measurements were recorded for each variable at each assessment time point and the mean values were used in the analyses.
Methacholine provocation test
A Wiesbadener Doppelinhalator (Wiesbadener Inhalatoren-Vertrieb, Wiesbaden, Germany) giving an output of 0.2 ml in 2 min was used for the methacholine provocation test, which was performed in accordance with American Thoracic Society guidelines [20]. sGaw was measured 30 and 90 s after completing each 2-min nebulization of increasing concentrations of methacholine dissolved in saline (doubling concentrations from 1 mg ml−1–256 mg ml−1, i.e. a total of nine administrations). The provocation test was performed at screening, baseline and 2, 6, 12 and 24 h post-administration, after the measurements of lung function were recorded. The concentration of methacholine resulting in a 35% decrease of sGaw from baseline was considered to be the provocative concentration (PC35 sGaw methacholine). If a decrease in sGaw of 35% was not achieved, the highest dose of methacholine, 256 mg ml−1, was considered to be the provocative dose.
Pharmacokinetics
Blood samples (5 ml) for pharmacokinetic assessments were collected in heparinized tubes containing the esterase inhibitor phenylmethylsulphonyl fluoride (PMSF) at pre-dose (baseline), 5, 15, 30 min and 1 and 3 h after every administration. Aclidinium and its acid and alcohol metabolites in plasma were determined by means of liquid chromatography/tandem mass spectrometry (LC/MS/MS). The lower limit of quantification (LLOQ) was 0.5 ng ml−1 for aclidinium and the alcohol metabolite, and 5 ng ml−1 for the acid metabolite. All analyses were performed at ADME Bioanalysis, Vergère (France).
Safety
At screening, all subjects underwent an examination consisting of a medical history, a physical examination (height, weight, calculation of BMI), recording of vital signs (blood pressure, heart rate, body temperature), ECG and laboratory assessments (haematology, biochemistry, serology, urine analysis and drug screen). At each treatment period, vital signs and an ECG were recorded immediately before drug administration and at several time points up to 24 h post-administration. Physical examination and laboratory assessments were also performed 24 h post-administration. All AEs occurring during the trial and any concomitant medications taken were recorded.
Statistical analyses
Drug activity was assessed by sGaw, Raw and PC35 (the concentration of methacholine required to reduce baseline sGaw by 35%).
Two study populations were described: the safety population (all subjects who received at least one dose of study medication) and the per-protocol population (all subjects in the safety population who completed at least one of the treatments according to the provisions of the protocol). The safety population was used in the analysis of demographic, pharmacokinetic and safety data while the per-protocol population was used in the analysis of pharmacological activity data. A sample size of 12 subjects was considered to be sufficient to meet the study objectives, based on the exploratory nature of the study. All calculations were performed using observed cases only and no imputation was carried out.
For all assessments, descriptive statistics were calculated by treatment and assessment time points. sGaw and Raw values are presented as absolute values and percentage of baseline values, and the area under the concentration time curve (AUC) over a 24 h period was also calculated. PC35 values are presented as PC35 doubling concentration units, calculated from log10(PC35)/[log10(2)]−1 and the AUC of PC35 doubling concentration units was calculated. Analysis of the absolute values of sGaw and Raw and of the PC35 doubling concentration units was carried out by means of an analysis of covariance (ancova) model for crossover designs including sequence, subjects within the sequence period, treatment, time and time-by-treatment interaction as factors, and the corresponding baseline values as covariates. sGaw and Raw, presented as percentage of baseline values, were analyzed by means of an anova model for crossover designs including sequence, subjects within the sequence period, treatment, time and time-by-treatment interaction as factors.
Results
Subject disposition
Twelve male subjects were randomized and 11 subjects completed the trial. There was one discontinuation due to intercurrent viral meningitis. There were no protocol deviations and all 12 subjects were included in the safety and per-protocol populations. None of the subjects required rescue medication with inhaled salbutamol during the methacholine provocation test.
Effect of aclidinium on airway calibre
All aclidinium doses significantly increased sGaw, both in terms of relative and absolute change, at all time points 1–24 h compared with placebo, except aclidinium 50 µg at 1 and 24 h (Table 2; Figure 1). The increases were greatest with the aclidinium 300 µg and 600 µg doses [average increases of 49.1% (0.38 l kPa−1 s−1) and 57.8% (0.44 l kPa−1 s−1), respectively; P < 0.0001]. Statistically significant differences were also observed between the 50 µg dose compared with the 300 µg and 600 µg doses [average increases of 28.8% (0.18 l kPa−1 s−1) and 37.5% (0.24 l kPa−1 s−1), respectively, P < 0.0001]. There was no significant difference between the 300 µg and 600 µg doses of aclidinium. Similar results were seen with sGaw AUC, with the mean (SE) differences from placebo of 4.89 (1.44), 8.47 (1.45) and 10.61 (1.53) l kPa−1 h for aclidinium 50 µg, 300 µg and 600 µg, respectively. All aclidinium doses were significantly greater than placebo (P= 0.0051 for the 50 µg dose and P≤ 0.0001 for the other two doses), with the 300 µg and 600 µg doses also being significantly greater than the 50 µg dose (P= 0.0276 and 0.0019, respectively). There was no significant difference between the 300 µg and 600 µg doses (P= 0.1764).
Table 2.
Mean values of specific airway conductance (sGaw, l/kPa/s) at all time points assessed
| Placebo (n= 9) | Aclidinium 50 µg (n= 9) | Aclidinium 300 µg (n= 9) | Aclidinium 600 µg (n= 8) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Time (h) | LSM | LSM | 95% CI | P | LSM | 95% CI | P | LSM | 95% CI | P |
| 1 | 0.99 | 1.03 | 0.83, 1.26 | 0.6424 | 1.40 | 1.15, 1.61 | <0.0001 | 1.34 | 1.00, 1.56 | 0.0001 |
| 2 | 1.03 | 1.24 | 1.00, 1.52 | 0.0085 | 1.40 | 1.20, 1.56 | <0.0001 | 1.53 | 1.20, 1.75 | <0.0001 |
| 4 | 0.98 | 1.19 | 0.93, 1.50 | 0.0073 | 1.40 | 1.15, 1.61 | <0.0001 | 1.41 | 1.15, 1.56 | <0.0001 |
| 6 | 1.03 | 1.33 | 0.99, 1.71 | 0.0003 | 1.46 | 1.26, 1.61 | <0.0001 | 1.46 | 1.08, 1.74 | <0.0001 |
| 8 | 0.85 | 1.12 | 0.86, 1.41 | 0.0010 | 1.34 | 1.07, 1.56 | <0.0001 | 1.33 | 1.07, 1.48 | <0.0001 |
| 12 | 1.03 | 1.28 | 1.04, 1.57 | 0.0015 | 1.42 | 1.11, 1.69 | <0.0001 | 1.52 | 1.13, 1.80 | <0.0001 |
| 24 | 0.95 | 1.08 | 0.94, 1.27 | 0.0842 | 1.11 | 0.91, 1.26 | 0.0236 | 1.34 | 0.92, 1.65 | <0.0001 |
LSM, least squares means.
Figure 1.
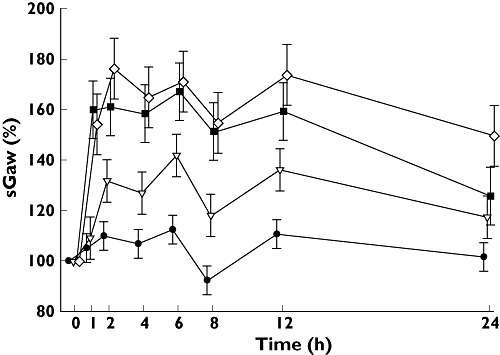
Mean (± SE) changes in specific airway conductance (sGaw, %) over 24 h as a percentage of baseline value. Placebo ( ); 50 µg aclidinium bromide (
); 50 µg aclidinium bromide ( ); 300 µg aclidinium bromide (
); 300 µg aclidinium bromide ( ); 600 µg aclidinium bromide (
); 600 µg aclidinium bromide ( )
)
A significantly greater reduction in Raw, in terms of both absolute and relative change (Figure 2), was seen with the aclidinium 300 µg and 600 µg doses compared with placebo at all time points (P < 0.04), except for the absolute change at the 24 h assessment for the 600 µg dose (P= 0.3694) and the relative change at the 24 h assessment for the 300 µg dose (P= 0.1230). Similar to the sGaw results, there was also a significant difference in the average Raw values between the 50 µg dose and the two higher doses (P < 0.003). Mean (SE) differences between treatment groups and placebo in Raw AUC over the 24 h period were −0.76 (0.068; P= 0.2761), −1.49 (0.68; P= 0.0368) and −2.46 (0.71; P= 0.0017) (kPa s−1 l−1) h for the aclidinium 50 µg, 300 µg and 600 µg doses, respectively.
Figure 2.
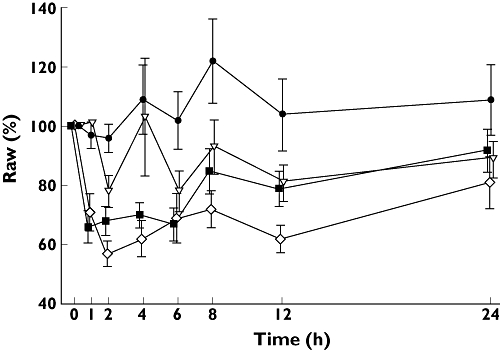
Mean (± SE) changes in airway resistance (Raw, %) over 24 h as a percentage of baseline value. Placebo ( ); 50 µg aclidinium bromide (
); 50 µg aclidinium bromide ( ); 300 µg aclidinium bromide (
); 300 µg aclidinium bromide ( ); 600 µg aclidinium bromide (
); 600 µg aclidinium bromide ( )
)
Effect of aclidinium on bronchial responsiveness to methacholine
To induce a decrease of ≥35% in sGaw, increasing the dose of aclidinium resulted in the use of higher concentrations of methacholine. This effect was more evident for the two highest aclidinium doses in which the highest dose of methacholine (256 mg ml−1) was unable to provoke responsiveness in some subjects. Statistically significant differences in PC35 doubling concentration units were seen between all three aclidinium doses and placebo (P= 0.0033 for the 50 µg dose and P < 0.0001 for the other two doses; Figure 3). There was also a statistically significant difference between aclidinium 50 µg and the two higher doses (P < 0.0001). In the analysis of AUC(0,24 h) PC35 doubling concentration units, statistically significant differences were seen between the two highest doses of aclidinium and placebo (P < 0.0001), as well as between the 50 µg dose and the two higher doses (P < 0.0001). However, there was no statistically significant difference between the aclidinium 50 µg dose and placebo (P= 0.1362).
Figure 3.
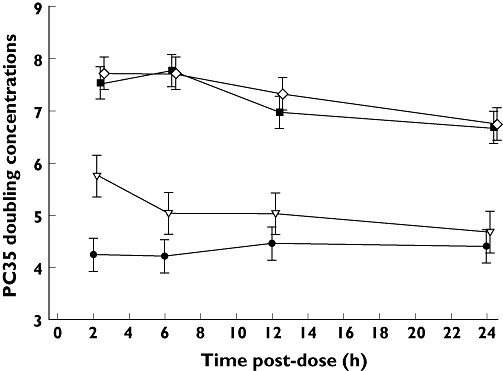
Effect of aclidinium on methacholine-induced bronchoconstriction, as measured by PC35 sGaw doubling concentrations (data shown as least squares means ± SE). Placebo ( ); 50 µg aclidinium bromide (
); 50 µg aclidinium bromide ( ); 300 µg aclidinium bromide (
); 300 µg aclidinium bromide ( ); 600 µg aclidinium bromide (
); 600 µg aclidinium bromide ( )
)
Pharmacokinetics
The concentration of aclidinium and its known metabolites were below the LLOQ in plasma at all time points. Therefore, no analyses were performed on the pharmacokinetic data for aclidinium or its metabolites.
Safety
Aclidinium was safe and well tolerated. Two subjects (one in each of the aclidinium 300 µg and 600 µg groups) experienced mild headache which, in both cases, resolved the same day following administration of 500 mg paracetamol. A third subject was hospitalized with a serious AE of viral meningitis with fever and headache, and was withdrawn from the study before the third treatment administration (aclidinium 600 µg). None of these events was considered to be related to aclidinium. There were no clinically significant effects on physical examination, vital signs, ECG or laboratory data.
Discussion
This phase I exploratory trial was the first study of aclidinium in humans. The study showed that the two higher doses of aclidinium investigated, 300 µg and 600 µg, provided the greatest improvements in sGaw and Raw, compared with placebo, from 1 h after administration up to 24 h. Smaller improvements were also observed with aclidinium 50 µg, the lowest dose evaluated in the study. Decreased bronchial responsiveness to methacholine was observed with all three doses of aclidinium compared with placebo, although the treatment effect, compared with placebo, was also greater with the two higher doses (300 µg and 600 µg). There was no significant difference between the two higher doses of aclidinium for sGaw, Raw or methacholine responsiveness. Effects on sGaw, Raw and methacholine responsiveness were sustained for at least 24 h (the last time point assessed) with the 300 µg and 600 µg doses.
The bronchodilatory activity of aclidinium and its ability to reduce methacholine-induced bronchoconstriction were demonstrated at the first time point assessed, 1 h after inhalation for sGaw and Raw, and 2 h for methacholine responsiveness. Further studies are required to establish in more detail the time to onset of action with aclidinium in humans and to explore the aclidinium profile in comparison with other bronchodilators.
Whole-body plethysmography was the method chosen to assess bronchodilator activity. A cholinergic tone is present in healthy subjects, and since it has been shown that increasing the number of sGaw readings reduces the variability and improves the sensitivity of the test, especially in healthy subjects [21], we used a mean value of five satisfactory measurements in this study. PC35 sGaw methacholine was selected to measure the prevention of methacholine-induced bronchoconstriction because, although sGaw and FEV1 changes correspond to one another during the methacholine provocation test, sGaw is considered to be a more sensitive measure of airway calibre than FEV1[22].
Pharmacokinetic analysis could not be performed because of the extremely low plasma concentrations of aclidinium and its metabolites. This is consistent with the rapid plasma clearance of aclidinium demonstrated in preclinical studies [14], and may explain the favourable tolerability of aclidinium observed in this study. It is important that anticholinergic agents have minimal systemic concentrations, as muscarinic receptors are widely distributed outside of the airways and lungs, particularly in the cardiovascular, gastrointestinal and central nervous systems [23].
Aclidinium has the potential to be a valuable new therapy for the treatment of COPD. Results from this phase I study strongly suggest that aclidinium is well tolerated and effective in improving airway conductance and in decreasing bronchial responsiveness. It offers significant bronchodilation and reduction in methacholine-induced bronchoconstriction soon after administration and sustained effects for at least 24 h. The results of this study in healthy subjects form the basis of future studies in patients with COPD.
Competing interests
The authors have the following potential competing interests to declare: Vanessa J. Schelfhout has received funding from Almirall for presenting a poster at ERS 2007. Pau Ferrer was an employee of Almirall until November 2001, and organized, wrote and managed the present clinical trial. Josep Maria Jansat, Francesc Peris and Esther Garcia Gil are employees of Almirall. Guy F. Joos has received funding from the following companies: GlaxoSmithKline (GSK), AstraZeneca, Boehringer Ingelheim and Novartis; lecture fees received from GSK, AstraZeneca and Boehringer Ingelheim; scientific advisory board fees received from GSK, AstraZeneca, Boehringer Ingelheim and Altana. All of these entities have an interest in therapies for COPD.
We would like to thank Mrs Vera Collart-Van de Velde (Lung Function Technician) for technical support in the performance of lung function testing. We thank Mark Hughes, PhD, from Complete Medical Communications, who provided medical writing support funded by Almirall, Barcelona, Spain.
REFERENCES
- 1.Rabe KF, Hurd S, Anzueto A, Barnes PJ, Buist SA, Calverley P, Fukuchi Y, Jenkins C, Rodriguez-Roisin R, van Weel C, Zielinski J. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD Executive Summary. Am J Respir Crit Care Med. 2007;176:532–55. doi: 10.1164/rccm.200703-456SO. [DOI] [PubMed] [Google Scholar]
- 2.Hogg JC. Pathophysiology of airflow limitation in chronic obstructive pulmonary disease. Lancet. 2004;364:709–21. doi: 10.1016/S0140-6736(04)16900-6. [DOI] [PubMed] [Google Scholar]
- 3.Hogg JC, Chu F, Utokaparch S, Wood R, Elliott WM, Buzatu L, Cherniack RM, Rogers RM, Sciurba FC, Coxson HO, Paré PD. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med. 2004;350:2645–53. doi: 10.1056/NEJMoa032158. [DOI] [PubMed] [Google Scholar]
- 4.Belmonte KE. Cholinergic pathways in the lungs and anticholinergic therapy for chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2005;2:297–304. doi: 10.1513/pats.200504-043SR. [DOI] [PubMed] [Google Scholar]
- 5.Caulfield MP, Birdsall NJ. International Union of Pharmacology. XVII. Classification of muscarinic acetylcholine receptors. Pharmacol Rev. 1998;50:279–90. [PubMed] [Google Scholar]
- 6.Coulson FR, Fryer AD. Muscarinic acetylcholine receptors and airway diseases. Pharmacol Ther. 2003;98:59–69. doi: 10.1016/s0163-7258(03)00004-4. [DOI] [PubMed] [Google Scholar]
- 7.Gross NJ, Co E, Skorodin MS. Cholinergic bronchomotor tone in COPD: estimates of its amount in comparison to that in normal subjects. Chest. 1989;96:984–7. doi: 10.1378/chest.96.5.984. [DOI] [PubMed] [Google Scholar]
- 8.Nisar M, Earis JE, Pearson MG, Calverley PM. Acute bronchodilator trials in chronic obstructive pulmonary disease. Am Rev Respir Dis. 1992;146:555–9. doi: 10.1164/ajrccm/146.3.555. [DOI] [PubMed] [Google Scholar]
- 9.Gross NJ. Ipratropium bromide. N Engl J Med. 1988;319:486–94. doi: 10.1056/NEJM198808253190806. [DOI] [PubMed] [Google Scholar]
- 10.Casaburi R, Mahler DA, Jones PW, Wanner A, San PG, ZuWallack RL, Menjoge SS, Serby CW, Witek T., Jr A long-term evaluation of once-daily inhaled tiotropium in chronic obstructive pulmonary disease. Eur Respir J. 2002;19:217–24. doi: 10.1183/09031936.02.00269802. [DOI] [PubMed] [Google Scholar]
- 11.Gross NJ. Tiotropium bromide. Chest. 2004;126:1946–53. doi: 10.1378/chest.126.6.1946. [DOI] [PubMed] [Google Scholar]
- 12.Engstrom CP, Persson LO, Larsson S, Ryden A, Sullivan M. Functional status and well being in chronic obstructive pulmonary disease with regard to clinical parameters and smoking: a descriptive and comparative study. Thorax. 1996;51:825–30. doi: 10.1136/thx.51.8.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Murray CJ, Lopez AD. Alternative projections of mortality and disability by cause 1990–2020: Global Burden of Disease Study. Lancet. 1997;349:1498–504. doi: 10.1016/S0140-6736(96)07492-2. [DOI] [PubMed] [Google Scholar]
- 14.Gavaldà A, Miralpeix M, Ramos I, Vilella D, Sentellas S, Alberti J, Ryder H, Beleta J. Aclidinium bromide, a novel muscarinic receptor combining long residence at M3 receptors and rapid plasma clearance. Eur Respir J. 2007;30(Suppl. 51):209S–210S. [Google Scholar]
- 15.Joos GF, Schelfhout VJ, Kanniess F, Ludwig-Sengpill A, Garcia Gil E, Montejo EM. Bronchodilator effects of aclidinium bromide, a novel long-acting anticholinergic, in COPD patients: a Phase II study. Eur Respir J. 2007;30(Suppl. 51):210S. [Google Scholar]
- 16.Almirall Internal Document. Four-week inhalation toxicity study in dogs. 1900. RCC 780388.
- 17.Almirall Internal Document. Four-week inhalation toxicity study in rats. 1900. RCC 780772.
- 18.Dubois AB, Botheho SY, Bedell GN, Marshall R, Comroe JH. A rapid plethysmographic method for measuring thoracic gas volume: a comparison with a nitrogen washout method for measuring functional residual capacity in normal subjects. J Clin Invest. 1956;35:322–6. doi: 10.1172/JCI103281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dubois AB, Botheho SY, Comroe JH. A new method for measuring airway resistance in man using a body plethysmograph: values in normal subjects and in patients with respiratory disease. J Clin Invest. 1957;35:327–34. doi: 10.1172/JCI103282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Crapo R, Casaburi R, Coates A, Enright PL, Hankinson JL, Irvin CG, MacIntyre NR, McKay RT, Wanger JS, Anderson SD, Cockcroft DW, Fish JE, Sterk PJ. Guidelines for methacholine and exercise challenge testing-1999. Am J Respir Crit Care Med. 2000;161:309–29. doi: 10.1164/ajrccm.161.1.ats11-99. [DOI] [PubMed] [Google Scholar]
- 21.Houghton CM, Woodcock AA, Singh D. A comparison of plethysmography, spirometry and oscillometry for assessing the pulmonary effects of inhaled ipratropium bromide in healthy subjects and patients with asthma. Br J Clin Pharmacol. 2005;59:152–9. doi: 10.1111/j.1365-2125.2004.02262.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Joos GF, Pauwels RA, Van der Straeten ME. The effect of nedocromil sodium on the bronchoconstrictor effect of neurokinin A in subjects with asthma. J Allergy Clin Immunol. 1989;83:663–8. doi: 10.1016/0091-6749(89)90080-8. [DOI] [PubMed] [Google Scholar]
- 23.Abrams P, Andersson KE, Buccafusco JJ, Chapple C, de Groat WC, Fryer AD, Kay G, Laties A, Nathanson NM, Pasricha PJ, Wein AJ. Muscarinic receptors: their distribution and function in body systems, and the implications for treating overactive bladder. Br J Pharmacol. 2006;148:565–78. doi: 10.1038/sj.bjp.0706780. [DOI] [PMC free article] [PubMed] [Google Scholar]


