Fig. 5.
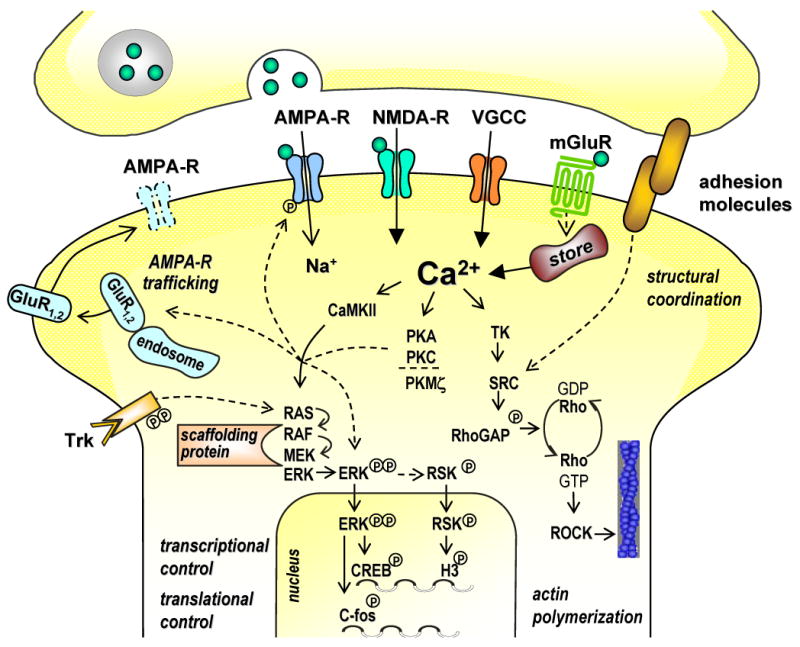
Molecular cascades of fear memory stabilization in the amygdala. A postsynaptic increase in intracellular Ca2+ concentration, mediated through Ca2+ influx via NMDA receptors and voltage-gated Ca2+ channels (VGCCs) and through release from intracellular stores upon activation of metabotropic glutamate receptors (mGluRs), triggers a plethora of signalling steps. Three major, mutually interconnected signalling routes involve Ca2+/calmodulin-dependent protein kinases II (CaMKII), the protein kinase (PK) family of enzymes, and tyrosine kinase (TK) pathways. Signalling cascades can reach the nucleus to induce macromolecular synthesis, and they can control translational processes. Consequently, they can act on cytoskeletal and adhesion molecules to re-organize and stabilize synaptic structures, or regulate AMPA receptor trafficking to the synapse. At intermediate steps, protein kinase signals converge on the mitogen-activated protein kinase (MAPK) signal transduction pathways, including the extracellular regulated kinases (ERK). RAS, RAF, and MEK kinases transduce intra- and extracellular signals, mediated for instance through tyrosin receptor kinases (Trk), to the MAPK/ERK pathway. Scaffolding proteins dictate specificity of activation as well as entry in the nucleus. MAPKs translocated into the nucleus phosphorylate transcription factors, such as cAMP response element binding protein (CREB). Actin rearrangement is under the control of RhoGTPases, whose activation from a GDP- to a GTP-bound form is controlled via Ca2+ or kinase pathways, including tyrosine kinases (TK) and SRC kinases. RhoGTPases control activity of Rho-associated kinases (ROCK), a key molecule for regulation of the cytosekeleton.
