Figure 4.
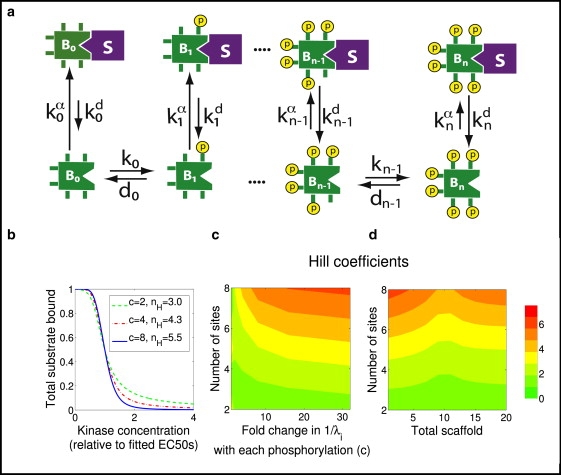
Strategy 4, regulation by release from sequestration. In this scenario, the sequestered form is the active (or inactive) entity, so the appropriate functional output is total substrate bound. (a) A scheme showing weaker binding with each phosphorylation. (b) Plot, assuming eight phosphosites (n = 8), showing how the fraction of B that is sequestered (total substrate bound) varies with the concentration of kinase A. For each value of c = λi−1/λi, the corresponding effective Hill number nH is also shown. Each curve is normalized by setting its EC50 equal to 1. (c) Hill coefficients as a function of the number of phosphorylation sites and fold change of binding ratios with each phosphorylation. (d) Hill coefficients as a function of the number of phosphorylation sites and total S, assuming the concentration of S is limiting (e.g., S is a protein and not a compartment).
