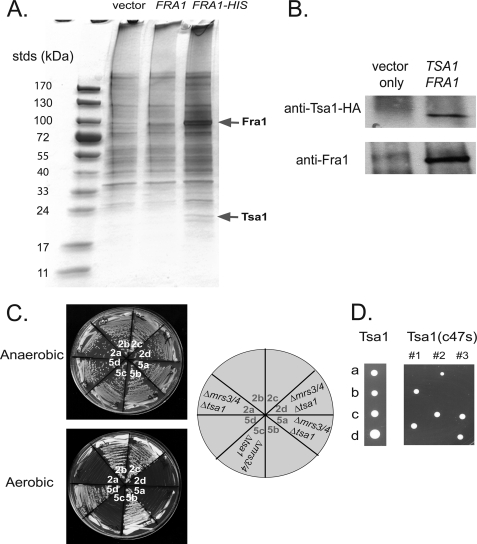
FIGURE 9.
Fra1 binds to Tsa1. A, wild type cells with empty vector, GAL1-regulated pFRA1, or GAL1-regulated FRA1-6XHIS were grown overnight in selective media, washed, and resuspended in 1.0% Triton X-100 plus 25 mm Tris-HCl, pH 7.4, with glass beads. Cells were vortexed for 10 min, centrifuged at 14,000 × g, and Fra1-His6 affinity-purified using nickel-nitrilotriacetic acid agarose according to the manufacturer's protocol. Eluted samples were run on SDS-PAGE and silver-stained. Protein bands were excised from the gel and analyzed by mass spectrometry. B, Δfra1 cells were transformed with GAL1 promoter pFRA1 and empty vector or pHA-TSA1. Cells were grown in galactose medium to induce the expression of Fra1, lysed as described in A, and immunoprecipitated for HA-Tsa1 with anti-HA antibody and protein A/G-agarose. Beads were boiled, and samples were run on SDS-PAGE followed by Western analysis probing with rabbit anti-HA and rabbit anti-Fra1 followed by peroxidase-conjugated goat-anti rabbit IgG. C, Δmrs3Δmrs4 diploid cells were deleted for TSA1 by deletion PCR as described under “Experimental Procedures.” Triple deletion diploids were dissected, and tetrads were grown on YPD in an anaerobic chamber. 2:2 segregation of tetrads was verified by the deletion markers. Two sets of tetrads (2 and 5) were grown on YPD for 2 days under aerobic or anaerobic conditions. D, diploid cells of Δmrs3Δmrs4Δtsa1 were either transformed with pHA-TSA1 or pHA-TSA1(c47s). After sporulation and dissection, tetrads (a, b, c, d spores) were grown on CM-URA plates at 30 °C for 3 days.