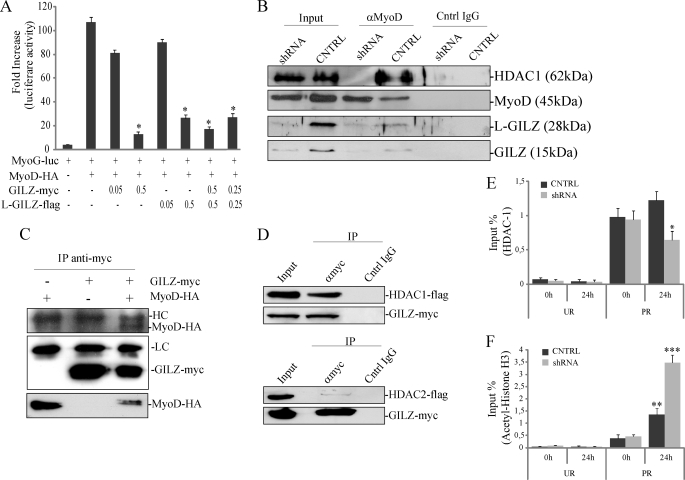
FIGURE 7.
GILZ and L-GILZ target MyoD transcriptional activity. A, MyoD transcriptional activity was measured with a luciferase reporter gene containing the myogenin promoter (MyoG-luc). *, p < 0.01 (n = 3). B, MyoD interacts with GILZ, L-GILZ, and HDAC1 in differentiating myoblasts. C2C12 myoblasts were cultivated in DM for 24 h before lysis. The cell lysates were subjected to immunoprecipitation with an anti-MyoD polyclonal antibody, and MyoD, GILZ, L-GILZ, and HDAC1 were identified by Western blot. C, GILZ directly interacts with MyoD. HEK293 cells were transfected with different combinations of plasmids encoding GILZ-Myc and MyoD-HA. GILZ-Myc was immunoprecipitated with an anti-Myc antibody, and co-immunoprecipitation was revealed with an anti-HA antibody. The bottom row represents MyoD-HA input. D, GILZ is able to interact with HDAC1 but not with HDAC2. HEK293 cells were co-transfected with expression vectors encoding GILZ-Myc and HDAC1-FLAG (upper panels) or HDAC2-FLAG (lower panels); immunoprecipitation was carried out with an anti-Myc antibody, and co-immunoprecipitation was revealed with an anti-FLAG antibody. E and F, for ChIP analysis, equivalent amounts of chromatin from same lysate used in endogenous IP assay described in B, control (CNTRL), and GILZ/L-GILZ silenced C2C12 myoblasts (shRNA) were immunoprecipitated in parallel with normal rabbit IgG and antibodies specific for HDAC1 (E) or acetylated H3 histones (F). The purified myogenin promoter then were analyzed by real time PCR. Bar graphs show the relative levels of bound DNA in HDAC1 (E) or acetylated H3 histones (F) immunoprecipitates, and results from three independent ChIP experiments combined are expressed as percentages of input chromatin, normalized to control IgG samples. UR, unrelated genomic region; PR, specific promoter region. *, p < 0.05 fourth column versus third column; **, p < 0.05 third column versus first column; ***, p < 0.05, fourth column versus third column (n = 3).