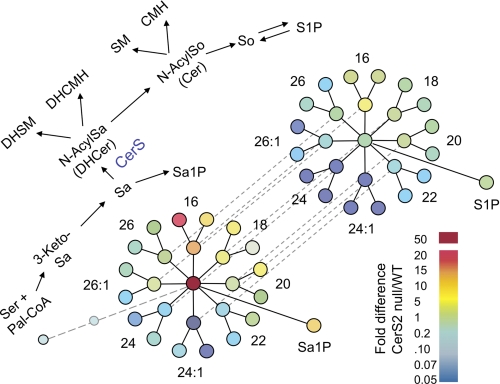
FIGURE 12.
Pathway relational map showing differences in the relative amounts of sphingolipids in 60-day-old CerS2 null versus WT mouse liver. The upper scheme (diagonal from lower left to upper right) summarizes the biosynthetic pathway of SLs beginning with condensation of serine and palmitoyl-CoA to 3-ketosphinganine (3-KetoSa) and then sphinganine (Sa), which is either N-acylated (to N-AcylSa, dihydroceramide (DHCer)) or phosphorylated (Sa1P). N-Acylated sphinganine can be desaturated to N-acylsphingosine (N-AcylSo, Cer), and both dihydroceramide and Cer can be converted into SM or glycosylated to ceramide monohexoses (CMH). Also shown is the hydrolysis of Cer to sphingosine (upper right), which can undergo phosphorylation to sphingosine 1-phosphate. Other intermediates and products of this scheme can also undergo turnover; some additional reactions (such as ceramide phosphate formation) are not shown because their amounts are small. The lower part of the figure depicts this pathway with all of the measured individual molecular subspecies as nodes that have been colored in the style of a heat map. The colors display the fold difference in the amounts of each compounds in CerS2 null versus WT mouse liver (i.e. CerS2/WT) using the color scale shown at the lower right. Thus, the light blue circle at the bottom left is for palmitoyl-CoA, followed by 3-ketosphinganine (3-keto-Sa) (which is shown smaller and faded to reflect that the amounts in both samples were too low for detection, but there was no evidence for accumulation of this intermediate in CerS2 null mouse), then by the node for sphinganine, which is deep red because sphinganine was substantially higher in the CerS2 null mice. Radiating from this hub are each N-acyl chain length metabolite of sphinganine (for examples, N-palmitoylsphinganine is labeled 16, and N-nervonoylsphinganine is labeled 24:1) (note that the former is elevated and the latter reduced), followed by the respective dihydrosphingomyelins (DHSM) (left nodes) and dihydroceramide monohexoses (DHCMH) (right nodes). Dashed lines relate each N-acyl chain length dihydroceramide to the respective Cer, which can be converted to SM and ceramide monohexose (outer nodes) or hydrolyzed to sphinganine (at the hub of this diagram). The phosphorylation products of sphinganine and So are also shown, labeled Sa1P and sphingosine 1-phosphate (S1P). The data used for this figure are from Tables 1 to 3. The layout of this scheme is from Ref. 9.