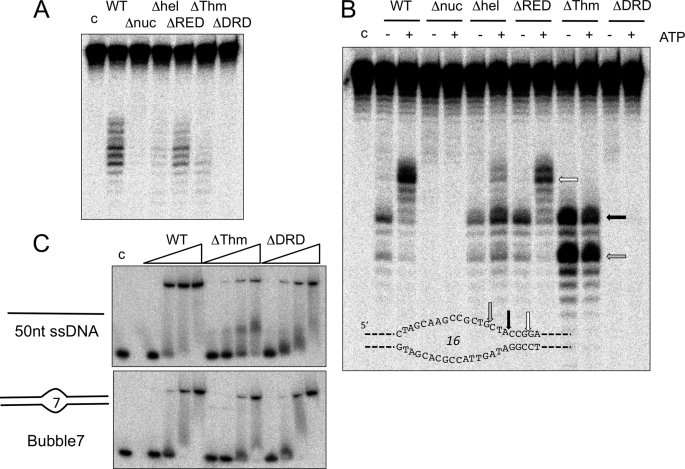
FIGURE 5.
Mutational analysis of XPB-Bax1 reveals subdomain function in binding and catalysis. A, activity of wild type (WT) and mutant versions of XPB-Bax1 on the Bubble 7 substrate in the presence of Mn2+ and ATP. B, activity of wild type and mutant versions of XPB-Bax1 on the Bubble 16 substrate in the presence of Mn2+, in the presence or absence of ATP. C, gel shift analysis of XPB-Bax1 binding to the Bubble 7 substrate and single-stranded DNA. The binding affinities of the wild type, ΔThm, and ΔDRD variants of XPB were compared by incubating 10 nm DNA with 50, 100, 250, and 500 nm XPB-Bax1. Lane c, DNA alone.