FIGURE 4.
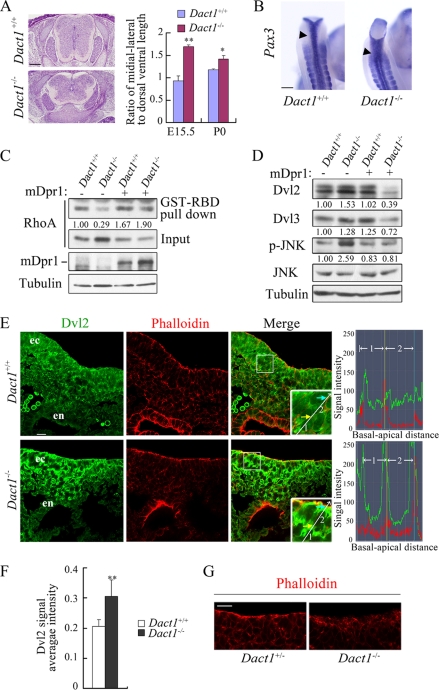
Deregulation of the PCP signaling is associated with altered Dvl activities in Dact1−/− mice. A, E15.5 wild-type and Dact1−/− embryos transversely sectioned through the lumbar. The neural tube was significantly wider in mutants than in wild-type siblings. At the E15.5 and postnatal day 0 stages, ratios of medial-lateral to dorsal-ventral axis were statistically analyzed in Dact1+/+ and Dact1−/− mice, respectively. **, p < 0.01; *, p < 0.05. More than four embryos were examined. B, malformed neural tube closure (arrowhead) is shown in E9.5 Dact1−/− embryos in the last several somites by whole mount in situ hybridization staining of Pax3 (dorsal view). C, decreased level of RhoA-GTP in Dact1−/− MEF as shown by pulldown assay. Glutathione S-transferase-Rho-binding domain, which specifically binds to RhoA-GTP (50 μg each), was added in equal cell lysates of Dact1+/+ and Dact1−/− MEF with or without Dact1 overexpression. After incubation in 4 °C for 1 h, RhoA was examined by anti-RhoA immunoblotting. Inputs (10% of total lysates) are shown in the lower panels. The endogenous protein levels of Dact1 were not detected because of the limited antibody sensitivity. The band density was quantitated by BandScan software (Glyko), and the relative density of pulled down RhoA was normalized to the total RhoA (input) in the same sample. D, MEF lysates (the same batch of cells prepared as in C) were subjected to immunoblotting with the indicated antibodies, and tubulin was used as a loading control. The relative density of Dvl2/3 in each lane was normalized to tubulin in the same sample, and the relative density of p-JNK was normalized by total JNK. E, E8.25 (5 somites) embryos were transversely sectioned at the primitive streak region, and indirect anti-Dvl2 immunofluorescence (green) was performed. Phalloidin stains F-actin (red). The images were taken by a Zeiss LSM 710 laser scanning microscope under identical illumination conditions and processed with Zen2009 software. Insets, a higher magnification highlights Dvl2 distribution patterns in ectoderm cells. A straight line indicates the direction from basal to apical side of two adjacent cells (designated as 1 and 2 sequentially), and signal intensity of Dvl2 (green) or phalloidin (red) along the line was shown in the right graphs. The yellow and blue arrows point to the cell boundary, and they correspond to the points shown as lines of the same colors in the right graphs. ec, ectoderm; en, endoderm. F, integral optical density of nine equal-sized squares along the ectoderm was measured in wild-type or Dact1−/− embryos, respectively, with Image-Pro Plus 6.0 software. Dvl2 signal average intensity = (integral optical density sum)/area. **, p < 0.01. G, phalloidin staining of F-actin in the primitive streak region of E8.5 (7–8 somites) embryos. Scale bars, 20 μm in E and G; 0.5 mm in A and B.