Abstract
Acipenser fulvescens, the lake sturgeon, belongs to one of the few extant non-teleost ray-finned (bony) fishes. The sturgeons (family Acipenseridae) have a phylogenetic history that dates back about 250 million years. The study reported here is the first investigation of peripheral coding strategies for spectral analysis in the auditory system in a non-teleost bony fish. We used a shaker system to simulate the particle motion component of sound during electrophysiological recordings of isolated single units from the eighth nerve innervating the saccule and lagena. Background activity and response characteristics of saccular and lagenar afferents (such as thresholds, response–level functions and temporal firing) resembled the ones found in teleosts. The distribution of best frequencies also resembled data in teleosts (except for Carassius auratus, goldfish) tested with the same stimulation method. The saccule and lagena in A. fulvescens contain otoconia, in contrast to the solid otoliths found in teleosts, however, this difference in otolith structure did not appear to affect threshold, frequency tuning, intensity- or temporal responses of auditory afferents. In general, the physiological characteristics common to A. fulvescens, teleosts and land vertebrates reflect important functions of the auditory system that may have been conserved throughout the evolution of vertebrates.
Keywords: auditory code, sturgeon, auditory, ancestral, particle motion, single unit
INTRODUCTION
The decomposition of sound into its frequency components through spectral analysis is common in all vertebrate auditory systems (Lewis and Fay, 2004). Spectral analysis of a sound signal reveals which frequencies are contained within a signal and the relative proportions (amplitudes) of the frequencies to one another. Spectral analysis is used for sound source segregation and the identification of sound sources, which are important for auditory scene analysis and communication (Bregman, 1990; Fay and Popper, 2000; Lewis and Fay, 2004). Detection of a signal of a particular frequency (or set of frequencies) that is embedded in broadband noise can be improved by passing it through a set of auditory filters which reduces the noise power while leaving the signal power largely unaltered (Popper et al., 2003).
Most studies on spectral analysis and the underlying peripheral coding mechanisms for frequency analysis in fish have been conducted in teleosts (derived bony fishes) (Furukawa et al., 1967; Fay, 1978a; Moeng and Popper, 1984; Fay and Ream, 1986; Fay, 1997; Fay and Edds-Walton, 1997a; Fay, 1998; Lu et al., 1998; Edds-Walton et al., 1999; McKibben and Bass, 1999; Fay, 2000; Weeg et al., 2002; Lu et al., 2003; Lu et al., 2004). Two studies were conducted in a cartilaginous fish species, Negaprion brevirostris (lemon shark) (Corwin, 1981a; Corwin, 1989) mainly measuring compound action potentials in the eighth nerve. However, there are no studies on the response properties of eighth nerve afferents (or any behavioral study) in a non-teleost bony fish. Such data would provide important insights into the evolutionary origin of coding mechanisms for sound in fishes or in tetrapods. One such species is Acipenser fulvescens (Rafinesque, 1817), the lake sturgeon, an ancestral member of the ray-finned fishes (or actinopterygians). Although the majority of actinopterygians are teleosts (more than 26,000 species) (Nelson, 2006) there are only 48 extant non-teleost actinopterygian species (Liem et al., 2001). Of these, 25 species are sturgeons (family: Acipenseridae) and two species belong to a sister group, the paddlefishes (family: Polyodontidae). Both Acipenseridae and Polyodontidae belong to the order Acipenseriformes, a group which has a phylogenetic history dating back about 250 million years (Grande and Bemis, 1996).
From an evolutionary perspective, studies on non-teleost actinopterygian groups are important as they provide insight into potential mechanisms of signal processing in an ancestral group. Moreover, such studies are critical for comparison with teleost studies since they help to distinguish which character traits are ancestral and which are derived (Wiley, 1981; Northcutt, 1985; Northcutt, 1986; Bolker, 2004) within the actinopterygians.
Acipenser fulvescens occur in the freshwaters of North America and Canada and usually lives on the bottom of the riverbed or lake. The fish migrate up rivers to spawn (Herald, 1961) and prefer certain spawning sites, which may be indicative of homing capabilities (Bemis and Kynard, 1997). Hearing may generally be useful in providing cues about the auditory scene (Fay and Popper, 2000), which then provides information that assist sturgeon in their migration between rivers and lakes either for spawning or feeding. Vocalizations by male A. fulvescens have been described during spawning season in the wild (Bruch and Binkowski, 2002). These sounds, however, have not been recorded and analyzed in terms of their frequency spectrum.
There have been a few physiological studies investigating hearing in ancestral groups of bony and cartilaginous fish (sharks and rays). These include one study on A. fulvescens and Polyodon spatula (Paddlefish) (Lovell et al., 2005) and several studies on sharks (e.g. Corwin et al., 1981b; Kenyon et al., 1998; Casper et al., 2003; Casper and Mann, 2007). All findings in these studies were based on auditory brainstem recordings (evoked potentials, AEPs). Other studies in sharks describe behavioral aspects of hearing (e.g. Banner, 1967; Kelly and Nelson, 1967; Myrberg et al., 1972; Nelson and Johnson, 1976). We decided to choose an ancestral bony fish instead of a cartilaginous fish since it makes a comparison with data obtained in teleosts (which we know most about) easier.
Most investigations on fish used loudspeakers which generated both pressure and particle motion. Although the inner ear of some fish species detect sound pressure indirectly, fish share a common mechanism for ear stimulation that involves one or more otolith organs (saccule, lagena, utricle) that respond directly, as inertial accelerometers, to acoustic particle motion accelerating the fish's body in a sound field (de Vries, 1950; Fay, 1984; Hawkins, 1993).
Particle motion can vary in unpredictable ways with respect to the sound pressure field generated by a loudspeaker immersed in a small tank. To avoid this problem, we used a shaker table to simulate the effect of particle motion by linearly accelerating the animal at different frequencies and intensities (Fay, 1984). This method has been previously used in only a few frequency studies in teleosts (e.g. Fay and Edds-Walton, 1997a; Weeg et al., 2002; Buchser et al., 2003; Lu et al., 2003; Lu et al., 2004).
In the current study we investigated the response of primary eighth nerve afferents innervating the saccule and lagena in A. fulvescens in terms of frequency selectivity, intensity dependence and temporal pattern. The data presented here represent the first detailed electrophysiological investigation of auditory coding strategies in a non-teleost bony fish.
MATERIALS AND METHODS
Experimental animals
Acipenser fulvescens, 25–37 cm in total length (21–28 cm in standard length) were obtained from the Wild Rose fish hatchery of the Wisconsin Department of Natural Resources. The fish were between 1 and 2 years old with body masses ranging between 45 and 115 g. They were housed in 45-gallon (~200 liter) tanks containing aerated water maintained by external filters and kept at a temperature of 17 to 18°C on a 12 h:12 h light:dark cycle. Recordings from 21 fish were used. We used the convention aa, bb, cc, etc. to identify specific animals, and numbers to identify units (e.g. ‘pp9’ stands for unit number 9, recorded from animal ‘pp’). The care and experimental use of A. fulvescens were carried out using protocols approved by the University of Maryland Institutional Animal Care and Use Committee.
Surgery and anesthesia
Fish were lightly anesthetized in a bath containing 0.05% buffered 3-aminobenzoic acid methane-sulfonate salt (Sigma, St Louis, MO, USA) dissolved in tank water, immobilized with an intramuscular injection of gallamine triethiodide in goldfish Ringer's solution (43 μg g−1 body mass), and then transferred to a surgical dish. For artificial respiration, aerated water was continuously pumped through the fish's mouth at a flow rate of 100–200 ml min−1. To locally anesthetize the surgical area, the skin at the dorsal surface of the head was dabbed with 2.5% Lidocaine (Astra Chemicals GmbH, Wedel, Germany). An opening into the cranium was made at the level of the entrance of the eighth nerve into the brain and from this moment the exposed brain was kept constantly moist with an inert fluorocarbon liquid (FC-77, 3M Corp, Sigma, St Louis, MO, USA) during the experiment. After completion of the surgery, fish were placed into an aluminum dish (diameter, 23 cm; height, 6 cm) which was mounted on a three-dimensional shaker table (described below). A rigid holder with a respirator tube especially designed for A. fulvescens was used to secure the head of the fish to the dish. Aerated tank water (temperature: 17–18°C) was pumped through the respirator into the fish's mouth via a Neslab RTE-111 chiller pump (Cole-Parmer, Vernon Hills, Il, USA).
In preparation for electrophysiological recordings from the posterior ramus of the eighth nerve (Fig. 1), the brain was slowly retracted to the left side with a custom-made plastic retractor. Special care was taken that semicircular canals and all otolith organs of the ear were not damaged during this procedure.
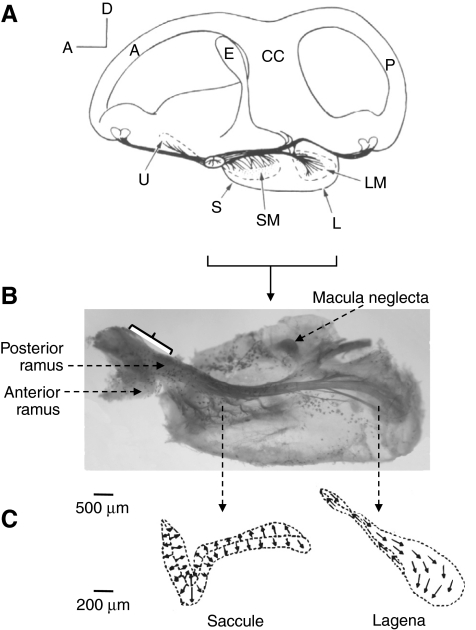
Fig. 1.
(A) Diagram of a medial view of the right ear showing the spatial orientation of the saccule and lagena (located vertically in one pouch) in Acipenser sturio (Baltic sturgeon) (Retzius, 1881). A, anterior semicircular canal; CC, crus commune; E, endolymphatic duct; L, lagena; LM, lagenar macula (epithelium); P, posterior semicircular canal; S, saccule; SM, saccular macula; U, utricle. (B) Innervation of the right saccule and lagena in Acipenser fulvescens (medial view; photograph by M. Meyer). The bracket indicates the recording site on the posterior ramus carrying fibers from the saccule and lagena. The more posterior portion of the nerve (just innervating the lagena) is tightly attached to the pouch making recordings difficult without damaging the pouch. (C) Outline of the saccule and lagena in A. fulvescens showing the orientation of the hair cells. Arrows are pointing in the direction of the kinocilium [adapted from Lovell et al. (Lovell et al., 2005)].
Stimulation system
The shaker table developed by Fay (Fay, 1984) was used to test frequency and intensity responses to sound in primary afferents of A. fulvescens simulating the effects of particle motion of underwater sound in a natural environment (for details, see Fay and Edds-Walton, 1997b; Lu et al., 2003; Lu et al., 2004). The table consisted of a circular dish driven by five shakers. Four shakers (Bruel and Kjaer no. 4810, Naerum, Denmark) generated motion in the horizontal plane, while the fifth (Bruel and Kjaer no. 4809, Naerum, Denmark) generated vertical motion. The horizontal shakers were arranged in two orthogonal pairs, each in a push–pull arrangement. The vertical shaker was attached via a rod to the center of the bottom of the dish. As a result of the shaker positions, the stimulus consisted of linear oscillatory motions of the circular dish, thereby moving the fish along distinct axes in the horizontal and midsagittal planes. Before measuring frequency responses, we determined the most common best direction of afferents.
The entire shaker system was positioned on a Micro-G pneumatic vibration isolation table (K&Us Equipment Inc, Boulder Creek, CA, USA). Signals to the front-to-back shaker pair, side-to-side shaker pair and the vertical shaker were digitally synthesized sinusoids (500 ms long) with 20 ms rise and fall times repeated eight times. Sinusoids were independently read out of the three channels of a 16 bit digital-to-analog converter (DA, Tucker Davis Technologies, Alachua, FL, USA) at a 10 kHz sampling rate. Each signal was low-pass filtered at 2 kHz, controlled in amplitude by a TDT PA4 programmable attenuator and amplified by a Techron power amplifier (Model 5507, AE Techron Inc., Elkhart, IN, USA). The amplified signals were further attenuated by 32 dB using a resistor network to improve signal-to-noise ratio at the shaker inputs.
Three accelerometers (PCB model 002A10, Flexcel, Piezotronics, Depew, NY, USA; sensitivity: 1 V g−1 of acceleration) positioned on the vertical, side-to-side and front-to-back axes were used to calibrate dish movement before and after the fish was positioned in the stimulus dish. The accelerometer output was monitored and used to make adjustments to the phases and amplitudes of the signals to create sinusoidal, linear movements along defined axes in the horizontal and vertical planes, at defined frequencies and intensities by use of a calibration program (Fay, 1984). Directional stimuli were presented at 100 Hz in six axes in the horizontal plane (90 deg., 60 deg., 30 deg., 0 deg., −30 deg. and −60 deg.) and six axes in the midsagittal plane (0 deg., 30 deg., 60 deg., 90 deg., 120 deg. and 150 deg.). Once it was determined that most afferents innervating the saccule or lagena in A. fulvescens responded best to vertical stimulation at or near 90 deg. elevation (M.M., unpublished data), all stimuli testing the frequency response were set at 90 deg. vertical.
Test frequencies ranged between 50 and 1000 Hz for the first stimulus protocol. Once it was determined that the upper frequency limit of afferents did not exceed 700 Hz, the frequency range was reduced to 50–700 Hz (50, 100, 141, 185, 244, 303, 409, 714). These frequencies were chosen so that there was an integer number of bins in the period histogram at each frequency, making vector strength calculations possible, and to capture a wide range of frequencies. For isolevel frequency response functions, this frequency–stimulus protocol was repeated for several stimulus levels within the unit's dynamic range.
Recording and data acquisition
Extracellular recordings were made using 2 mol l−1 NaCl-filled glass pipettes with resistances between 20 and 50 MΩ. Tip resistances were achieved using a micropipette puller (P97, Sutter Instrument Co., Novato, CA, USA). The micropipette was positioned on the nerve under visual control and then advanced through with a remote hydraulic micro-drive. The tip of the reference electrode (a silver–silver-chloride wire of 1 mm diameter) was placed in the fluid of the brain cavity. Neural signals were amplified using a DAM 80 amplifier (World Precision Instruments, Sarasota, FL, USA) and band pass filtered between 300 and 3000 Hz. Spikes were discriminated using a single voltage criterion which means that one threshold criterion well above noise levels was used to create a TTL pulse for each action potential. Spike times were recorded with a 0.1 ms sampling period.
Data analysis
Data from frequency–responsive units were analyzed with respect to some or all of the following parameters: background rate, threshold, degree of phase locking, best frequency (BF, determined from isolevel frequency response functions), characteristic frequency (CF, determined from tuning curves), bandwidth (Q10 dB), response-level functions and temporal response pattern.
From the spike times recorded during stimulation, period histograms (PHs), peristimulus time histograms (PSTHs), and inter spike interval histograms (ISIHs) were formed and the coefficient of synchronization (R) was calculated to measure the degree of phase locking (Goldberg and Brown, 1969). To minimize misinterpretations of R when having a smaller number of spikes (Ns), the Rayleigh statistic Z=R2Ns was applied to quantify phase locked responses (Batschelet, 1981; Fay, 1984).
Background activity was defined as spontaneous firing of spikes by units in the absence of an intentional stimulus and was measured over eight repetitions of the stimulus interval with no stimulus present. Threshold was determined at 90° vertical (best direction for most units) and at 100 Hz (BF for most units) and defined as the stimulus displacement level (dB re. 1 nm) which corresponds to Z=20 (the probability of obtaining a Z-value of 20 or more by chance is less than 0.001).
To quantify response strength, both spike rate and the computed Z-value were plotted initially. However, it was decided to focus on the Z-value as a measure of response strength for the data presented in this paper since most units showed background activity and 99% of the units were strongly phase locked (with R-values >0.5 at 10–15 dB above threshold).
To investigate coding strategies to sound intensity, response (Z)–intensity functions for which stimulus intensity was expressed as dB re.1 nm displacement r.m.s. (root mean square) were plotted. Response–intensity functions were fitted with a sigmoidal curve and the maximum slope (which occurs at the midpoint of the function) was chosen for quantification. Tuning curves and contour plots were created from isolevel frequency response functions by linear interpolation between levels. To assess the sharpness of a frequency response, Q10 dB (dividing the CF by the bandwidth at 10 dB above threshold) was determined for V-shaped tuning curves (N=36). We chose Q10 dB since this measure of bandwidth is widely used in the vertebrate literature (e.g. Kiang et al., 1965).
To compare BF distributions of A. fulvescens with data obtained in teleost species, afferents with certain BFs were added for each species and expressed as a percentage of the total number of afferents tested. This cumulative percentage would then be plotted as a function of BF resulting in one graph for each species (data points were connected for the purpose of clarity). Frequencies at which most afferents had their BF would show the steepest part of the function.
RESULTS
Spatial orientation of otolith organs and innervation in A. fulvescens
We present a description of the innervation and spatial orientation of otolith organs (Fig. 1) to illustrate certain differences from teleost species used for single unit studies from the eighth nerve in previous studies. The saccule and lagena in A. fulvescens are located in one vertically oriented pouch whereas the utricle is oriented horizontally (Fig. 1A). The eighth nerve exits the brain and branches off into two major rami. The anterior ramus innervates the utricle and the anterior and horizontal cristae, whereas the posterior ramus innervates the saccule, lagena, macula neglecta and the posterior crista (Fig. 1B). The posterior ramus runs parallel to the saccule and lagena. At the level of the saccule, the ramus sends off many short branches, which innervate hair cells of the saccule (Fig. 1B). More posteriorly the ramus divides into two major branches closely attached to the pouch, one of them innervating the posterior crista, the other innervating the lagena and macula neglecta. Recordings were made from the part of the posterior ramus of the right ear located most proximal to the brain (Fig. 1B). It was impossible to access any distal portions of this ramus for recordings without severely damaging the saccule and lagena because of its tight connection with the pouch containing those epithelia.
A differentiation between afferents innervating either the saccule or the lagena based on hair cell orientation and/or spatial location of these epithelia was not possible since the hair cells on the saccule and the lagena are oriented in various directions on each epithelium (Lovell et al., 2005) (Fig. 1C) and both epithelia are oriented in the midsaggital plane of the fish. Thus, to estimate the proportion of data from afferents projecting to one or the other of these organs requires verification through labeling studies. We attempted extracellular injections with neurobiotin into the recording site in several animals but did not obtain enough data to make a statistical comparison. However, the injections showed at least one afferent innervating the saccule and one innervating the lagena in two different animals.
Physiology
We recorded and analyzed 170 sound-responsive single units from 21 animals. All of these units responded to sound with an increased spike rate and by phase-locking to the stimulus. About 90 additional units encountered did not respond to the stimulus (‘non-responding’ units).
During recordings, the electrode was always positioned over the dorsal surface of the right posterior ramus. While advancing the electrode from dorsal to ventral through the nerve branch, non-responding units were found more dorsally, whereas responding units were found more ventrally.
Background activity and thresholds
Background rates of 159 responding units ranged from 0 to 142 spikes s−1 with a mean of 23 spikes s−1. Most of these afferents (91%, 145/159) showed background activity. Three general patterns of background firing occurred: units with irregular intervals between spikes (44%), units that had regular spike trains with intervals distributed around a maximum peak (32%) and units showing a bursting pattern of background activity with ISIHs having multiple peaks (21%). Background rates available for 23 non-responding units ranged between 9 and 44 spikes s−1 with a mean of 20 spikes s−1. The firing rate of most of those units did not exceed 22 spikes s−1 (82%, 18/23).
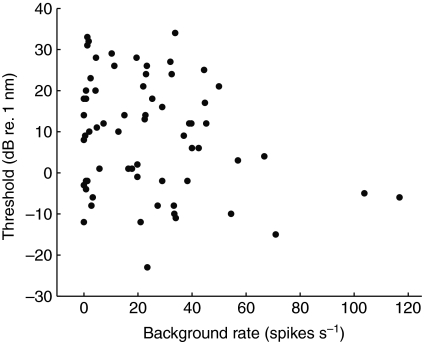
Thresholds (determined for 65 units at their BF, which was 100 Hz) ranged from −23 dB to 34 dB (re. 1 nm) with a mean of 9 dB, corresponding to displacement values of 0.07 nm (minimum), 2.8 nm (mean) and 50 nm (maximum). A correlation between threshold and spontaneous rate was significant when considering the entire population of afferents shown (including the afferents firing at rates higher than 100 spikes s−1; r=−0.25, N=65, P=0.05, two-tailed test). However, there was no correlation when the two afferents with high background rates were excluded from the data set (r=−0.17, N=63, P=0.05, two-tailed test; Fig. 2).
Fig. 2.
Background rate versus threshold for 65 afferents evaluated. There was no correlation (r=−0.17, N=63, P=0.05) between these two variables when the two afferents with spike rates higher than 100 spikes s−1 were excluded from the statistical evaluation. With the two units included, the correlation was significant (r=−0.25, N=65, P=0.05).
Frequency characteristics: isolevel frequency response functions and tuning curves
Units differed in their BF (or CF), in the sharpness of tuning and in the shape of the frequency function (Fig. 3). For example, some tuning curves either had a symmetric V shape (e.g. ff11 and qq5, Fig. 3) or had a slightly sharper cutoff towards lower frequencies than toward higher frequencies (unit ss11, Fig. 3) or vice versa. In most units, BF or CF as well as the shape of the frequency function stayed consistent across levels.
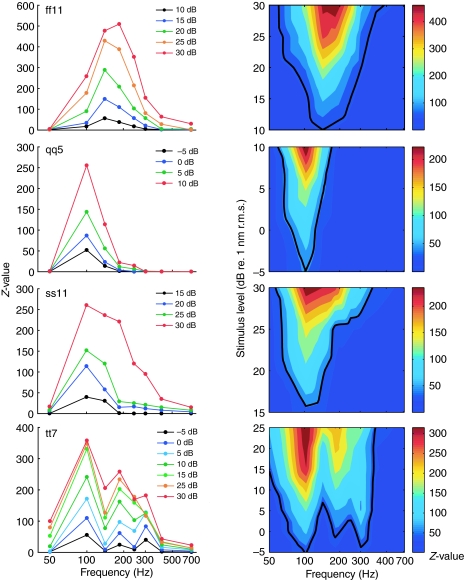
Fig. 3.
Frequency responses of four units. The left column represents isolevel frequency responses: Z-value is plotted as a function of frequency for different levels (see inset; x-axis: logarithmic scale; data points connected by straight lines for clarity). The right column shows contour plots: stimulus level is plotted as a function of frequency (x-axis: logarithmic scale). The black line in each contour plot outlines the tuning curve (criterion: Z=20). The color code to the right of each contour plot indicates Z-values. Frequency responses of afferents differed in terms of BF (or CF), in the sharpness of tuning and the shape of the function. ff11: BF/CF=141 Hz, Q10 dB=0.57, background rate=15 spikes s−1. qq5: BF/CF=100 Hz, Q10 dB=1.3, background rate=20 spikes s−1. ss1: BF/CF=100 Hz, Q10 dB=0.83, background rate=43 spikes s−1. tt7 had three peaks at 100, 185 and 303 Hz, Q10 dB was not defined, background rate=54 spikes s−1. Nineteen percent of units had a more complex response profile with two or three peaks (e.g. unit tt7).
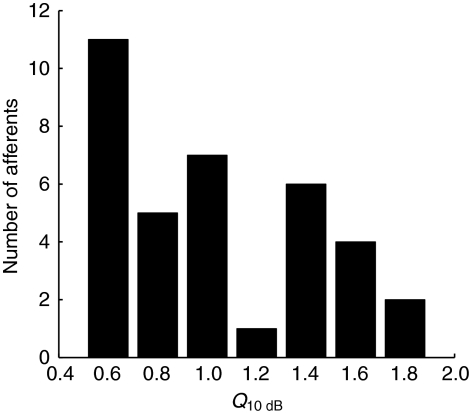
Some units (19%; 33/170 units) had a second or third peak in their isolevel frequency response function (called a ‘multi-peak function’; unit tt7, Fig. 3) in contrast to 81% (137/170) with only one peak (a ‘mono-peak function’; units ff11, qq5, ss11, Fig. 3). These multi-peak units obtained from 12 different animals were always found mixed together with the majority of afferents having mono-peak response profiles. The response versus level function for unit tt7 (Fig. 4A), as well as the PSTH (Fig. 4B), ISIH (Fig. 4C) and PH (Fig. 4D) obtained at 100 Hz resembled those of single unit recordings from afferents with mono-peak functions. Thus, unit tt7 was most probably a single-unit recording and the multiple peaks were not caused by a second afferent from a multi-unit recording. Plotting the isolevel frequency function of unit tt7 as spike rate versus frequency instead of Z-value versus frequency resulted in similar multi-peaked profiles (not shown). Q10 dB values computed for 36 tuning curves from mono-peak units ranged between 0.6 and 1.8 (1.06±0.42; mean ± s.d.; Fig. 5).
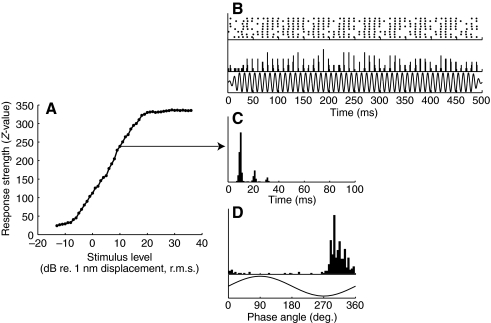
Fig. 4.
Response characteristics for unit tt7. (A) Response versus level function of unit tt7 obtained at 100 Hz (data points connected by straight lines). The function for this unit was similar to response–level curves obtained for mono-peak functions. (B) Response pattern shown in raster plot (top) and peri stimulus time histogram (middle; bin size: 1 ms). The unit fired bursts in a continuous fashion throughout stimulus presentation (100 Hz-stimulus shown in bottom trace). (C) Inter spike interval histogram (bin size: 1 ms). (D) Period histogram (bin size: 5 deg.). All histograms have been obtained at 10 dB re. 1 nm displacement and at 100 Hz.
Fig. 5.
Sharpness of frequency tuning for 36 afferents as determined from their Q10 dB values. Q10 dB values were similar to those found for afferents with low CFs (<400 Hz) in other vertebrates.
Distribution of best frequencies and comparison with teleost species
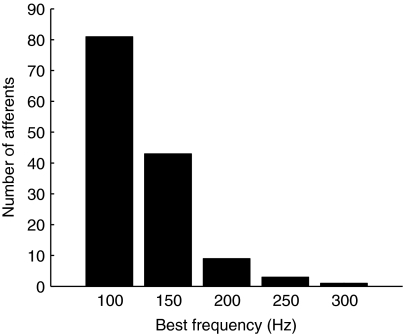
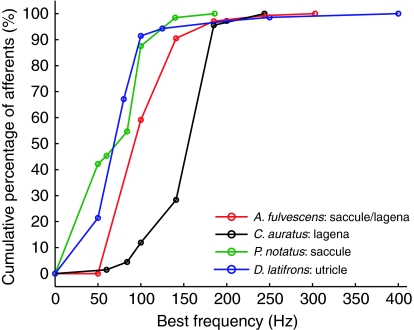
The majority of afferents in A. fulvescens (59%; 81 out of 137 afferents) had BFs at 100 Hz, whereas 31% (43/137) had their BF at 141 Hz (Fig. 6). Best frequencies of only 10% (13/137) of the afferents exceeded 141 Hz with one afferent having a BF at 303 Hz (the maximum BF found). The BF distribution for saccular and lagenar afferents of A. fulvescens appears to be similar to the BF distribution for the saccular afferents in Porichthys notatus [plainfin midshipman, green line, Fig. 7; modified from Weeg et al. (Weeg et al., 2002)] and utricular afferents in Dormitator latifrons [sleeper goby, blue line, Fig. 7; modified from Lu et al. (Lu et al., 2004)] and less similar to lagenar afferents in Carassius auratus [goldfish, black line, Fig. 7 (Meyer et al., 2004)] since most afferents had their BF near 200 Hz in the lagena of C. auratus. The BF distribution for utricular afferents is similar to data obtained for saccular and lagenar afferents in D. latifrons according to Lu et al. (Lu et al., 2004), however, the actual distribution values were only published for utricular afferents (Lu et al., 2004). In A. fulvescens no afferents were found that showed a stronger response at 50 Hz than at frequencies between 100 and 150 Hz.
Fig. 6.
Distributions of best frequencies (BFs) of 137 mono-peak functions in A. fulvescens. Test frequencies were 50, 100, 141, 185, 244, 303, 409 and 714 Hz. Best frequency was defined as the frequency that elicited the greatest response at the lowest stimulus level presented. Most afferents responded best at 100 Hz.
Fig. 7.
Comparison of best frequency (BF) distributions determined for eighth nerve afferents between A. fulvescens and teleost species stimulated using the shaker table. For each species, the cumulative percentage of afferents was computed and plotted as a function of BF (y-axis shows the percentage of afferents having their BF up to and including the value shown on the x-axis). Red: saccular and lagenar afferents (combined) in A. fulvescens (N=137); black: lagenar afferents in C. auratus (N=67) (Meyer et al., 2004); green: saccular afferents in P. notatus (N=64) (Weeg et al., 2002); blue: utricular afferents in D. latifrons (N=70) (Lu et al., 2004). Data points are joined through straight lines for clarity.
Displacement, velocity and acceleration tuning curves
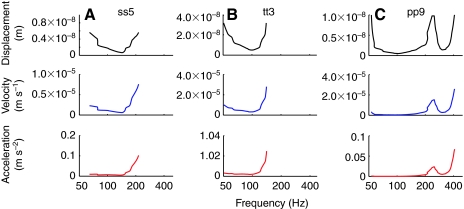
The tuning curves (obtained at the stimulus level in dB re. 1 nm displacement) of several units with different shapes and CFs were converted into dB re. 1 nm s−1 velocity and dB re. 1 nm s−2 acceleration as shown in Fig. 8. The outcome of this conversion was that no matter which shape the tuning curve had when converted to acceleration, all units essentially became low-pass filters with corner frequencies that corresponded to the BF. The sensitivity to frequencies higher than the corner frequency decreased very rapidly. This is the expected result if the saccule and/or lagena were acceleration-sensitive end organs because an acceleration-sensitive transducer generally has a constant response to accelerations at frequencies below a critical frequency. The unit shown in Fig. 8A (ss5) had its BF around 150 Hz and shows a substantially flat response towards frequencies smaller than 150 Hz and steeply decreased at frequencies higher than BF when plotted as dB re 1 nm s−2 acceleration. A similar change in shape was observed for unit tt3 (Fig. 8B) which had its BF at 100 Hz. Unit pp9 (Fig. 8C) had a W-shaped tuning curve and the tuning curve profile became less prominent when plotted as dB re. 1 nm s−2 acceleration but did not disappear completely. For this unit there was a slight increase in threshold seen at 50 Hz compared to 100 Hz.
Fig. 8.
Tuning curves of units ss5 (A), tt3 (B) and pp9 (C) plotted as dB re. 1 nm displacement (top), dB re. 1 nm s−1 velocity (middle) and as dB re. 1 nm s−2 acceleration (bottom) versus frequency (x-axis: logarithmic scale). Data points are connected by straight lines in each graph. All units essentially became low-pass filters when expressed in terms of acceleration. This is the expected result if the saccule and/or lagena were acceleration-sensitive end organs. Unit pp9 differed slightly from the expected pattern, showing a slight increase in threshold at 50 Hz compared with 100 Hz.
Level encoding
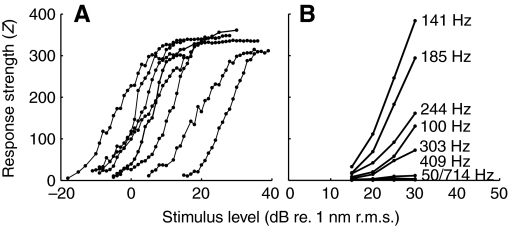
Response (Z) versus intensity functions obtained at 100 Hz were sigmoid shaped with dynamic ranges extending from 9 to 24 dB (re. 1 nm displacement) and slopes varying between 14 and 28 Z/dB. Functions differed widely in threshold (Fig. 9A). When considering the variations of response–level functions of one unit with frequency as shown in Fig. 9B, it becomes apparent that the slope is steepest at BF and becomes flatter with increasing or decreasing frequencies relative to BF.
Fig. 9.
(A) Response (Z) versus level functions obtained at best frequency (BF) of seven units. Units had a wide variety of thresholds and functions were monotonically increasing up to a saturation point. (B) Response–level functions of unit ff3 (BF=141 Hz) plotted for various frequencies. Points are connected by straight lines in each graph for clarity. The steepest slope occurs at the BF, and steepness decreases with deviation from BF (at frequencies lower, as well as higher than BF).
Temporal encoding and effect of level on firing pattern
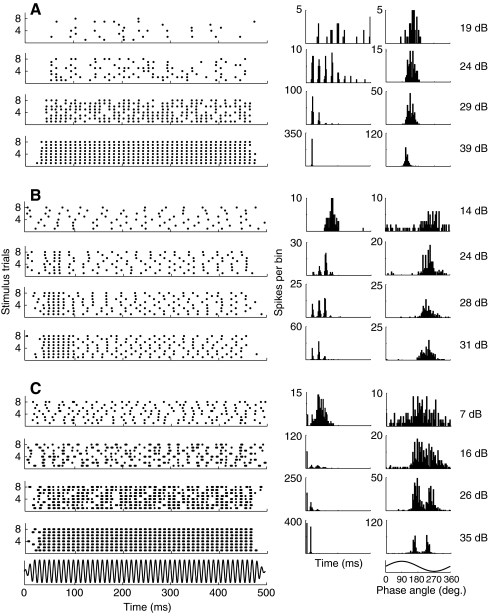
How units respond to stimulus duration is revealed by PST histograms (PSTHs) and raster plots. We have plotted the raster plot, inter spike interval distribution (ISIH) and the period histogram (PH) to 100 Hz stimulation for 53 units (from 17 animals) to investigate the firing pattern over the course of stimulus presentation and to reveal the effect of stimulus level on the pattern and phase locking. Three different response patterns were found (Fig. 10). Most units (85%, 45/53) fired continuously and in a highly phase-locked manner throughout the stimulus (‘tonic firing pattern’, Fig. 10A, unit uu8). Uu8 showed strong phase locking that increased at higher levels and the phase angle decreased slightly with level.
Fig. 10.
Response patterns of saccular and lagenar afferents in A. fulvescens to sinusoidal stimulation [obtained at best frequency (BF)=100 Hz]. Left to right: raster plots, inter spike interval histograms (bin size: 1 ms) and period histograms (bin size: 5 deg.). Each graph is presented at four different stimulus levels to illustrate the effect of level on the response pattern for each unit. (A) Tonic response pattern (background rate: 0 spikes s−1; threshold: 18 dB re. 1 nm). (B) Phasic tonic response pattern (background rate: 23 spikes s−1; threshold: 13 dB re. 1 nm). (C) Phase locked bursting (background rate: 43 spikes s−1; threshold: 6 dB re. 1 nm).
Fifteen percent of the afferents (8/53) fired more spikes during the onset of the stimulus (‘phasic tonic response pattern’). This response pattern remained consistent across levels (example shown in Fig. 10B, unit pp7). The phasic component becomes proportionally stronger with increasing stimulus intensity and this unit also showed strong phase-locking (see distinct peaks in its ISIH related to the period of the stimulus waveform and single peak in its PH).
Some units firing in a tonic or phasic manner showed bursting (15%, 8/53) that increased with level. These bursts were usually double spikes occurring at a very short interval even at low levels (Fig. 10C, unit ss10) near threshold. The PHs show two peaks at higher levels that become more and more distinct with increasing level. Thus, both spikes were phase-locked and occurred at different phase angles but they were not separated by 180 deg.
DISCUSSION
The unique aspect about A. fulvescens for studies of sensory coding is its cladistic position within the vertebrates and its early occurrence in the evolutionary history of vertebrates. In this study, we characterized basic auditory afferent response properties in a manner directly comparable with previous studies in teleosts and other vertebrates.
Comparison to teleosts and other vertebrates
Background rates as well as physiological response characteristics such as threshold, frequency response, bandwidth, response to stimulus intensity and temporal response showed many similarities with data from teleosts and other vertebrates as discussed in the following section.
Background rates and thresholds
Saccular and lagenar afferents in A. fulvescens showed high variability in background firing (ranging from 0 to 150 spikes s−1) and in firing pattern, being either regular, irregular (most fibers) or bursting [as also seen in Gadus morhua, Atlantic cod (Horner et al., 1981); Ictalurus punctatus, channel catfish (Moeng and Popper, 1984); C. auratus (Fay and Ream, 1986); O. tau (Fay and Edds-Walton, 1997a); P. notatus (McKibben and Bass, 1999); D. latifrons (Lu et al., 1998)]. Thresholds also varied widely with displacements, ranging between 0.07 nm and 50 nm, r.m.s., which is equivalent to results found for saccular or lagenar afferents in C. auratus (Fay, 1984; Fay and Ream, 1986; Meyer et al., 2004), O. tau (Fay and Edds-Walton, 1997a) and D. latifrons (Lu et al., 1998). Such a large range of thresholds means that that sounds of widely different levels can be encoded by the auditory periphery. The correlation between threshold and spontaneous rate remains ambiguous, however. Units with very high background rates (larger than 100 spikes s−1) are rarer and were not recorded from damaged fibers, therefore they were included in the dataset and not treated as outliers. However, more afferents of this category are needed to determine if there is indeed a correlation between background rate and threshold.
Tuning and sharpness
Shape and sharpness of tuning curves or isolevel frequency functions among afferents varied and were similar to those seen in teleosts that resemble band pass filters. Although the tuning curves of most units were V-shaped (as is typical for auditory systems in vertebrates), some units (19%) showed W-shaped tuning curves and isolevel frequency functions with more than one peak (max. three peaks). Those complex profiles were also found in teleosts [e.g. saccular afferents of P. notatus (Weeg et al., 2002); medullar neurons of O. tau (Edds-Walton and Fay, 2003); utricular afferents of D. latifrons (Lu et al., 2004)] stimulated with the shaker system and for saccular afferents in P. notatus stimulated by a loud speaker (McKibben and Bass, 1999). Such complexity might arise from complex interactions of peripheral tuning mechanisms such as ion-channel properties, hair cell resonances or micromechanics.
Q10 dB values determined for 36 units (with V-shaped tuning curves) in A. fulvescens resembled those values of afferents with low CFs (<400 Hz) of mammals, reptiles and birds and of afferents innervating the caudal auditory papillar regions or the basilar papilla in frogs. This could mean that the existence of lower frequency tuning curves might have been conserved throughout the evolution of vertebrates (possibly a more ancestral characteristic of auditory coding) and the mechanism for lower frequency tuning might be shared.
Best frequencies
Saccular and lagenar afferents in A. fulvescens had similar BFs to teleost species having a BF distribution between 50 and 400 Hz when tested with the same stimulation method (Fay and Edds-Walton, 1997a; Lu et al., 2003; Lu et al., 2004; Weeg et al., 2002). Those teleosts all appear to have no direct connection between a gas-filled structure and the ear (as seen in teleost species belonging to the Otophysi and Mormyridae). In A. fulvescens the swim bladder is not in close proximity to the ear either (M.M., unpublished data). The only potential difference between A. fulvescens and teleosts was that no afferents were found that had a stronger response at 50 Hz than at frequencies between 100 and 150 Hz. Similar experiments conducted at frequencies lower than 50 Hz and at intermediate frequencies between 50 and 100 Hz would be useful to further investigate if there are lower frequency fibers (<100 Hz) in the saccule and lagena of A. fulvescens.
Experiments in the shark N. brevirostris using a loudspeaker suspended in air (Corwin, 1981a) demonstrated similar BFs ranging between 40 and 400 Hz (best responses between 90 and 150 Hz). It would be interesting to perform single unit recordings in N. brevirostris or other cartilaginous fish species using a shaker table for easier interpretation of threshold data and better comparability to physiological experiments in teleosts.
Level encoding
Response–level functions in A. fulvescens, were sigmoid shaped and monotonic, and dynamic ranges of response–level functions were comparable to data from saccular afferents in O. tau (Fay and Edds-Walton, 1997a) and lagenar and utricular afferents in D. latifrons (Lu et al., 2003; Lu et al., 2004). Generally, the dynamic ranges of individual afferents seemed to be smaller in fish than for some auditory afferents in other vertebrates [e.g. max. 40 dB in mammals (Kiang et al., 1965); max. 40 dB in reptiles (Eatock et al., 1991); max. 50 dB in frogs (Simmons et al., 2007)]. However, the wide variation in thresholds for saccular and lagenar afferents (representing a range of at least 60 dB re 1 nm displacement) may be enough to compensate for the limited dynamic range of individual fibers as a population. A sigmoidal, monotonic shape as opposed to a non-monotonic function seen in some vertebrates [e.g. mammals (Kiang et al., 1965)] decreases ambiguity and is thus optimal for coding intensities by a population of afferents. Thus, saccular and lagenar afferents in A. fulvescens are well suited to encode the intensity of sound via population coding because the afferent thresholds cover a wide range of levels and because of the monotonic shape of input–output functions.
Temporal encoding
Units in A. fulvescens showed a variety of response patterns similar to those found in teleost auditory afferents (e.g. Lu et al., 2003; Lu et al., 2004). Most units in A. fulvescens showed a tonic response pattern over the entire duration of the stimulus presentation that did not change with level (Fig. 10A). Units with phasic tonic (or adapting) response patterns were also present (Fig. 10B), as is the case for auditory afferents of terrestrial vertebrates (Ruggero, 1992; Gleich and Manley, 2000). A few afferents in A. fulvescens showed phase-locked bursting similar to lagenar and utricular afferents of D. latifrons (Lu et al., 2003; Lu et al., 2004). In summary, there is remarkable variability in temporal response pattern both in A. fulvescens and in teleosts (e.g. Lu et al., 2003; Lu et al., 2004) possibly reflecting the importance of encoding different temporal aspects of sounds, such as the duration of sound (encoded by tonic firing) or the onset of sound (encoded by phasic-tonic or adapting fibers).
Any potential difference in BF distribution or other physiological characteristics between A. fulvescens and teleosts might be associated with the difference in otolith structure in A. fulvescens. The ears in A. fulvescens contain otoconia that appear to be compressed or loosely distributed particles, in contrast to dense and solid otoliths in teleosts (Calström, 1963; Popper, 1978; Lychakov, 1995; Gauldie, 1996). However, all physiological characteristics such as thresholds, the shape, bandwidths and variations of frequency functions, intensity responses and temporal responses are remarkably similar to those found in teleosts. This suggests that the otoconia in sturgeon and the solid otolith in teleosts fulfill similar functions in terms of hearing.
Non-responding units
The posterior ramus of the eighth nerve innervates the saccule, lagena, posterior canal and the macula neglecta. Since neither the macula neglecta nor the posterior crista contain otoliths or otoconia, we assumed that they did not respond to linear acceleration. Therefore, all afferents that showed frequency tuning (all ‘responding units’) were considered to receive their input from the saccule or the lagena. The non-responding fibers (35%) might instead have innervated the macula neglecta, posterior crista or belonged to efferents. Canal afferents in O. tau have a mean background rate of about 50 spikes s−1 ranging between 0.7 and 134 spikes s−1 (Boyle and Highstein, 1990). Thus, the spike rates found in non-responsive afferents of A. fulvescens (range: 9–44 spikes s−1) may be from fibers innervating the posterior canal, although mean rates were lower than for O. tau (20 spikes s−1). Background rates of efferents projecting to the auditory or vestibular periphery in teleosts vary between species. Mean rates in D. latifrons were 14.86 spikes s−1 (Tomchick and Lu, 2006). In C. auratus (Hartmann and Klinke, 1980) and in O. tau (Highstein and Baker, 1985; Edds-Walton et al., 1999) mean rates did not exceed 10 spikes s−1. Background rates for afferents innervating the macula neglecta are not known.
Temporal versus spike rate coding
Generally, spike rate as well as Z (phase-locked spike rate) showed similar trends when plotted against frequency. Afferents showed robust phase-locking within the physiological frequency detection range in A. fulvescens. Such a code based on timing between spikes (temporal code) has been found to be sufficient to encode for behaviorally relevant frequencies as well as the temporal fine structure of sound stimuli in modern teleost fish (Fay and Coombs, 1983; Fay, 1978b). Moreover, a temporal code has been found in all vertebrates for acoustic waveforms and can now be confirmed for the most ancestral bony fish species studied so far. Thus, temporal encoding may represent the most ancestral neural code for hearing, although experiments in other ancestral bony fish as well as cartilaginous fish species are needed to confirm this hypothesis.
In addition to a temporal code there may also be a rate code since primary auditory afferents in A. fulvescens and teleosts are frequency tuned, based on spike rate. The mechanisms underlying such frequency tuning as well as the differences in filter-shape and bandwidth seen in primary otolithic afferents in fish are not well understood. However, they may arise from hair cell resonance (Sugihara and Furukawa, 1989; Steinacker and Romero, 1992) and micromechanical mechanisms between hair cells and their attachment to the otolithic membrane (Fay, 1997). Evidence for a place-frequency code in fish is unclear since only very few studies have suggested that response characteristics of different saccular or lagenar regions might vary (Furukawa and Ishii, 1967; Sand and Michelsen, 1978; Enger, 1981; Fay, 1997). Yet, even without a place code, the diversity in shape, bandwidth and sensitivity of filters in A. fulvescens and teleosts may be sufficient to encode intensity and frequency information. A given sound would create a specific signature or pattern across a population of afferents (population code) which could then be decoded centrally. The activation of only a few afferents differing in CF and bandwidth may be enough to encode a particular frequency by central neurons, for example the dominant frequency of vocalizations. In summary, both a temporal code as well as a spike rate code may be relevant for A. fulvescens and teleost species.
Displacement, velocity and acceleration tuning curves
When the tuning curves were plotted using acceleration rather than displacement as the stimulus parameter they appeared nearly flat at the lower frequencies. The corner frequency (corresponding to BF or CF) marks the frequency range up to which the fiber is most sensitive to stimulus acceleration. This tuning curve shape is consistent with the idea that the otolith organ is a damped simple harmonic oscillator driven by an external input, and the output of the organ is proportional to the acceleration of the otolith. At low frequencies, the output displacement depends only on the magnitude (A) of acceleration, thus the system acts like a pure accelerometer (stiffness controlled). At intermediate frequencies, the output displacement is proportional to A/ω (where ω is the input frequency), thus the system acts like a velocity detector (since this corresponds to the process of integration going from acceleration to velocity). The system at this point is predominantly controlled by viscous drag (acting against the moving mass). At higher frequencies the output displacement instead is proportional to A/ω2 (corresponding to the process of integration going from acceleration to displacement), thus the system becomes a displacement detector (mainly controlled by the inertia of the overlying mass).
The examples shown for A. fulvescens (Fig. 8) were representative of all tuning curves obtained from afferent responses. From these observations one may conclude that otolith organs in fish, including those carrying otoconia instead of otoliths (such as the saccule and lagena in A. fulvescens), principally act as accelerometers. The conversion to velocity or displacement tuning curves results in a V-shaped function with a ‘best’ frequency equal to the corner frequency (frequency at which the threshold begins to rise) of the acceleration tuning curves. Given velocity or displacement tuning curves, one is faced with the task of explaining the ‘best’ frequency and ‘frequency tuning’, whereas with acceleration tuning curves, the critical feature is only the corner frequency (bandwidth), or the frequency above which sensitivity begins to decline. Thus, units of acceleration seem to result in a simpler description of transducer behavior than either velocity or displacement. In this study, displacement manipulation was chosen simply because displacement units are more intuitive and understandable than either velocity or acceleration.
The conversion to acceleration tuning curves can reveal in addition, whether there may be mechanisms differing from accelerometers (such as velocity or displacement detection mechanisms). If such differences (e.g. pp9, Fig. 8C) occurred, one could then sample at finer frequencies and combine physiological studies with single unit labeling studies to correlate shape and corner frequency of acceleration tuning curves with different areas on the epithelium (e.g. areas having varying densities of otoconia) to reveal differences in micromechanics. In any case, a similar investigation at a finer frequency resolution and including lower frequencies (down to infrasound) might be useful for further investigations.
The role of the saccule and the lagena in hearing
The saccule and lagena in A. fulvescens are likely to be involved in hearing since tuning curves appeared more like band pass filters (when plotted with respect to displacement) and all physiological characteristics showed strong resemblance to data on the saccule in teleost fishes (Fay, 1984; Fay and Edds-Walton, 1997a; Fay and Edds-Walton, 1997b; Weeg et al., 2002; Buchser et al., 2003), which is considered to be involved in hearing. Possibly, otolith organs in fishes have a dual function, both vestibular (responding to gravitational stimuli and linear acceleration caused by self-induced motion) and auditory [linear acceleration caused by movement of the fish in the sound field (Platt, 1989) (reviewed by Popper et al., 2003)]. Generally, there was no dichotomy in the data for A. fulvescens that would suggest a functional separation between the saccule and lagena. Although the best frequencies did not vary much between otolith organs in D. latifrons (the only species for which frequency data of all three otolith organs are known), it may still be useful to measure the response properties of utricular afferents in A. fulvescens to investigate the potential role of the utricle for auditory (and vestibular) function.
Evolutionary considerations
This is the first study of the response properties of primary auditory afferents in a non-teleost bony fish that focuses on a detailed physiological analysis of frequency and intensity coding. Recordings from primary afferents innervating the saccule and lagena in A. fulvescens showed many similarities to the findings described for the auditory (or vestibular) periphery in teleost fishes and to auditory systems in other vertebrates as discussed above. The similarities to teleosts are interesting considering that A. fulvescens belongs to a group of fishes that had their major radiation approximately 250 million years ago. The similarities to other vertebrates (and teleosts) is also interesting when assuming, as a first approximation, that the coding strategies found in A. fulvescens may represent the ancestral condition for all vertebrates, at least as far back as the origin of the bony fishes. However, more species of the group of non-teleost bony fish are necessary to make a full assessment of ancestral versus more modern coding strategies (within the group of bony fishes), since A. fulvescens may have developed modern characteristics during its own evolutionary history.
Ultimately it is necessary to compare results from ancestral bony fish with those of members of the outgroup of bony fish, the cartilaginous fishes. There are similarities in terms of BF distribution between sharks (based on one single unit study by Corwin, 1981a) and A. fulvescens supporting that the data in sturgeon may reflect the ancestral condition in vertebrates (not just of bony fish), yet more detailed studies in cartilaginous fishes using the same stimulation method would be helpful to further evaluate this hypothesis.
In general, it seems likely that similarities between vertebrates occurred because the auditory system in vertebrates derived from a common ancestor. The assumption is based on the notion that certain auditory and vestibular structures are considered homologous, such as the vertebrate hair cell (Coffin et al., 2004) and that the gross morphology of the inner ears is very similar among vertebrates. The earliest vertebrates for which a sensitivity to sound of the inner ear was found were elasmobranches (Löwenstein and Roberts, 1950; Myrberg et al., 1972; Nelson and Johnson, 1976; Corwin, 1981a; Corwin, 1981b), thus it seems plausible that elasmobranches have similar coding strategies to ancestral bony fish and teleosts. A sensitivity to sound in Agnatha still remains to be investigated.
ACKNOWLEDGEMENTS
This research was supported in part by the NIH (NIDCD) R01 research grant R01 DC006215 to R. R. Fay and the Parmly Hearing Institute of Loyola University Chicago and by a P30 grant (P30 DC004664) to the Center of Comparative and Evolutionary Biology of Hearing of the University of Maryland. We thank the Parmly Hearing Institute for providing a Parmly Hearing Scholar fellowship to M. M. during a three months stay in Chicago. Lake sturgeons were kindly supplied by the Wisconsin Department of Natural Resources. Many thanks to Kaushik Ghose for help with Matlab and to Ruth Anne Eatock for comments on the manuscript. Finally we would like to thank the two anonymous reviewers for their suggestions. Deposited in PMC for release after 12 months.
Footnotes
- BF
- best frequency
- CF
- characteristic frequency
- ISIH
- interspike interval histogram
- PH
- period histogram
- PSTH
- peristimulus time histogram
REFERENCES
- Banner A. (1967). Evidence of sensitivity to acoustic displacements in the lemon shark, Negaprion brevirostris (Poey). In Lateral Line Detectors (ed. Cahn P. H.), pp. 265-273 Bloomington: Indiana University Press; [Google Scholar]
- Batschelet E. (1981). Circular Statistics in Biology New York: Academic Press; [Google Scholar]
- Bemis W. E., Kynard B. (1997). Sturgeon rivers: an introduction to acipenseriform biogeography and life history. Environ. Biol. of Fishes 48, 167-183 [Google Scholar]
- Bolker J. (2004). Embryology. In Sturgeon and Paddlefish of North America (ed. Le Breton G. T. O., Beamish F. W. H., McKinley R. S.), pp. 134-144 New York: Springer-Verlag; [Google Scholar]
- Boyle R., Highstein S. M. (1990). Efferent vestibular system in the toadfish: action upon horizontal semicircular canal afferents. J. Neurosci 10, 1570-1582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bregman A. (1990). Auditory Scene Analysis: The Perceptual Organization of Sound Cambridge, MA: MIT Press; [Google Scholar]
- Bruch R., Binkowski F. P. (2002). Spawning behavior of lake sturgeon (Acipenser fulvescens). J. Appl. Ichthyol. 18 570-579 [Google Scholar]
- Buchser W. J., Lu Z., Xu Z., Evoy W. H. (2003). Frequency response of saccular afferents in a teleost fish. Soc. Neurosci. Abstr. 93, 7 [Google Scholar]
- Carlström D. (1963). A crystallographic study of vertebrate otoliths. Biol. Bull. 125, 441-463 [Google Scholar]
- Casper B. M., Mann D. A. (2007). Dipole hearing measurements in elasmobranch fishes. J. Exp. Biol. 210, 75-81 [DOI] [PubMed] [Google Scholar]
- Casper B. M., Lobel P. S., Yan H. Y. (2003). The hearing sensitivity of the little skate, Raja erinacea: comparison of two methods. Environ. Biol. Fishes 68, 371-379 [Google Scholar]
- Coffin A. B., Kelley M., Manley G., Popper A. N. (2004). Evolution of sensory hair cells. In Evolution of the Vertebrate Auditory System (ed. Eatock R. A., Popper A. N., Fay R. R.), pp. 27-54 New York: Springer-Verlag; [Google Scholar]
- Corwin J. T. (1981a). Peripheral auditory physiology in the lemon shark: evidence of parallel otolithic and non-otolithic sound detection. J. Comp. Physiol. 142, 379-390 [Google Scholar]
- Corwin J. T. (1981b). Audition in elasmobranchs. In Hearing and Sound Communication in Fishes (ed. Tavolga W. N., Popper A. N., Fay R. R.), pp. 81-102 New York: Springer Verlag; [Google Scholar]
- Corwin J. T. (1989). Functional anatomy of the auditory system in sharks and rays. J. Exp. Zool. 2, 62-74 [Google Scholar]
- de Vries H. L. (1950). The mechanics of the labyrinth otoliths. Acta Oto-Laryngol. 38, 262-273 [DOI] [PubMed] [Google Scholar]
- Eatock R. A., Weiss T. F., Otto K. L. (1991). Dependence of discharge rates on sound pressure level in cochlear nerve fibers of the alligator lizard: implications for cochlear mechanisms. J. Neurophys. 65, 1580-1597 [DOI] [PubMed] [Google Scholar]
- Edds-Walton P. L., Fay R. R. (2003). Directional selectivity and frequency tuning of midbrain cells in the oyster toadfish (Opsanus tau). J. Comp. Physiol. 189, 527-543 [DOI] [PubMed] [Google Scholar]
- Edds-Walton P. L., Fay R. R., Highstein S. M. (1999). Dendritic arbors and central projections of physiologically characterized auditory fibers from the saccule of the toadfish (Opsanus tau). J. Comp. Neurol. 411, 212-238 [PubMed] [Google Scholar]
- Enger P. S. (1981). Frequency discrimination in teleosts – central or peripheral? In Hearing and Sound Communication in Fishes (ed. Tavolga W. N., Popper A. N., Fay R. R.), pp. 243-255 New York: Springer Verlag; [Google Scholar]
- Fay R. R. (1978a). Coding of information in single auditory nerve fibers of the goldfish. J. Acoust. Soc. Am. 63, 136-146 [DOI] [PubMed] [Google Scholar]
- Fay R. R. (1978b). Phase-locking in goldfish saccular nerve fibers accounts for frequency discrimination capacities. Nature 275, 320-322 [DOI] [PubMed] [Google Scholar]
- Fay R. R. (1984). The goldfish ear codes the axis of particle motion in three dimensions. Science 225, 951-953 [DOI] [PubMed] [Google Scholar]
- Fay R. R. (1997). Frequency selectivity of saccular afferents of the goldfish revealed by revcor analysis. In Diversity in Auditory Mechanics (ed. Lewis E. R., Long G. R. R., Lyon R. F., Narins P. M, Steele C. R., Hecht-Poinar E.), pp. 69-75 Singapore: World Scientific Publishers; [Google Scholar]
- Fay R. R. (1998). Auditory stream segregation in goldfish (Carassius auratus). Hear. Res. 120, 69-76 [DOI] [PubMed] [Google Scholar]
- Fay R. R. (2000). Spectral contrasts underlying auditory stream segregation in goldfish (Carassius auratus). J. Assn. Res. Otolaryngol. 1, 120-128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fay R. R., Coombs S. (1983). Neural mechanisms in sound detection and temporal summation. Hear. Res. 10, 69-92 [DOI] [PubMed] [Google Scholar]
- Fay R. R., Edds-Walton P. L. (1997a). Directional response properties of saccular afferents of the toadfish (Opsanus tau). Hear. Res. 111, 1-21 [DOI] [PubMed] [Google Scholar]
- Fay R. R., Edds-Walton P. L. (1997b). Diversity in frequency response properties of saccular afferents of the toadfish (Opsanus tau). Hear. Res. 113, 235-246 [DOI] [PubMed] [Google Scholar]
- Fay R. R., Popper A. N. (2000). Evolution of hearing in vertebrates: the inner ears and processing. Hear. Res. 149, 1-10 [DOI] [PubMed] [Google Scholar]
- Fay R. R., Ream T. J. (1986). Acoustic response and tuning in saccular nerve fibers of the goldfish (Carassius auratus). J. Acoust. Soc. Am. 79, 1883-1895 [DOI] [PubMed] [Google Scholar]
- Furukawa T., Ishii Y. (1967). Neurophysiological studies on hearing in goldfish. J. Neurophysiol. 30, 1377-1403 [DOI] [PubMed] [Google Scholar]
- Gauldie R. W. (1996). Fusion of otoconia: a stage in the development of the otolith in the evolution of fishes. Acta. Zool. 77, 1-23 [Google Scholar]
- Gleich O., Manley G. (2000). The hearing organ of birds and Crocodilia. In Comparative Hearing: Birds and Reptiles (ed. Dooling R. J., Popper A. N., Fay R. R.), pp. 70-138 New York: Springer Verlag; [Google Scholar]
- Goldberg J. M., Brown P. B. (1969). Response of binaural neurons of dog superior olivary complex to dichotic tonal stimuli: some physiological mechanisms of sound localization. J. Neurophysiol. 32, 613-636 [DOI] [PubMed] [Google Scholar]
- Grande L., Bemis W. E. (1996). Interrelationships of Acipenseriformes, with comments on Chondrostei. In Interrelationships of Fishes (ed. Stianssny M. L. J., Parenti L. R., Johnson G. D.), pp. 85-115 San Diego: Academic Press; [Google Scholar]
- Hartmann R., Klinke R. (1980). Efferent activity in the goldfish vestibular nerve and its influence on afferent activity. Pflueger's Arch. 38, 123-128 [DOI] [PubMed] [Google Scholar]
- Hawkins A. D. (1993). Underwater sound and fish behavior. In Behavior of Teleost Fishes (ed. Pitcher T. J.), pp. 129-169 New York: Chapmann and Hall; [Google Scholar]
- Herald E. S. (1961). Living Fishes of the World Garden City, New York: Doubleday and Company Inc. [Google Scholar]
- Highstein S. M., Baker R. (1985). Action of the efferent vestibular system on primary afferents in the toadfish, Opsanus tau. J. Neurophys. 54, 370-384 [DOI] [PubMed] [Google Scholar]
- Horner K., Hawkins A. D., Fraser P. J. (1981). Frequency characteristics of primary auditory neurons from the ear of the cod (Gadus morhua). In Hearing and Sound Communication in Fishes (ed. Tavolga W. N., Popper A. N., Fay R. R.), pp. 223-241 New York: Springer-Verlag; [Google Scholar]
- Kelly J. C., Nelson D. R. (1967). Hearing thresholds in the horn shark, Heterodontus francisci. J. Acoust. Soc. Amer. 58, 905-909 [DOI] [PubMed] [Google Scholar]
- Kenyon T. N., Ladich F., Yan H. Y. (1998). A comparative study of hearing ability in fishes: the auditory brainstem response approach. J. Comp. Physiol. A 182, 307-318 [DOI] [PubMed] [Google Scholar]
- Kiang N. Y. S., Watanabe T., Thomas C., Clark L. F. (1965). Discharge Patterns of Single Fibers in the Cat's Auditory Nerve Cambridge, MA: MIT Press; [Google Scholar]
- Lewis E. R., Fay R. R. (2004). Environmental variables and the fundamental nature of hearing. In Evolution of the Vertebrate Auditory System (ed. Manley G. A., Popper A. N., Fay R. R.), pp. 27-54 New York: Springer-Verlag; [Google Scholar]
- Liem K. F., Bemis W. E., Walker W. F., Jr, Grande L. (2001). Functional Anatomy of the Vertebrates. An Evolutionary Perspective, 3rd edn.Orlando: Harcourt College Publishers; [Google Scholar]
- Lovell J. M., Findlay M. M., Moate R. M., Nedwell J. R., Pegg M. A. (2005). The inner ear morphology and hearing abilities of the paddlefish (Polyodon spathula) and the lake sturgeon (Acipenser fulvescens). Comp. Biochem. Physiol. A 142, 286-296 [DOI] [PubMed] [Google Scholar]
- Löwenstein O., Roberts T. D. M. (1950). The equilibrium function of the otolith organs of the thornback ray (Raja clavatra). J. Physiol. 110, 392-415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Z., Popper A. N. (1998). Morphological polarizations of sensory hair cells in the three otolith organs of a teleost fish: fluorescent labeling of ciliary bundles. Hear. Res. 12, 47-57 [DOI] [PubMed] [Google Scholar]
- Lu Z., Xu Z., Buchser W. J. (2003). Acoustic response properties of lagenar nerve fibers in the sleeper goby (Dormitator latifrons). J. Comp. Physiol. A 189, 889-905 [DOI] [PubMed] [Google Scholar]
- Lu Z., Xu Z., Buchser W. J. (2004). Coding of acoustic particle motion by utricular fibers in the sleeper goby (Dormitator latifrons). J. Comp. Physiol. A 190, 923-938 [DOI] [PubMed] [Google Scholar]
- Lychakov D. V. (1995). Investigation of the otolithic apparatus in the Acipenser fry. J. Evol. Biochem. Phys. 31, 333-341 [Google Scholar]
- McKibben J. R., Bass A. H. (1999). Peripheral encoding of behaviorally relevant acoustic signals in a vocal fish: single tones. J. Comp. Physiol. 184, 563-576 [DOI] [PubMed] [Google Scholar]
- Meyer M., Popper A. N., Fay R. R. (2004). Frequency tuning and directional preferences in lagenar nerve fibers of the goldfish (Carassius auratus). Abst. Assn. Res. Otolaryngol. 27, 325 [Google Scholar]
- Moeng R., Popper A. N. (1984). Auditory responses of saccular neurons of the catfish (Ictalurus punctatus). J. Comp. Physiol. 155, 615-624 [Google Scholar]
- Myrberg A. A., Jr, Ha S. J., Walewski S., Banbury J. C. (1972). Effectiveness of acoustic signals in attracting epipelagic sharks to an underwater sound source. Bull. Mar. Sci. 22, 926-949 [Google Scholar]
- Nelson J. S. (2006). Fishes of the World New York: John Wiley; [Google Scholar]
- Nelson J. S., Johnson R. H. (1976). Some recent observations on acoustic attraction of Pacific reef sharks. In Sound Reception in Fishes (ed. Schuijf A., Hawkins A. D.), pp. 229-239 Amsterdam: Elsevier; [Google Scholar]
- Northcutt R. G. (1985). The brain and sense organs of the earliest vertebrates: reconstruction of a morphotype. In Evolutionary Biology of Primitive Fishes (ed. Foreman E. R., Gorbman A., Dodd J. M., Olsson R.), pp. 81-112 New York: Plenum Press; [Google Scholar]
- Northcutt R. G. (1986). Strategies of comparison: how do we study brain evolution? Verh. dt. zool. Ges. 79, 91-103 [Google Scholar]
- Platt C., Popper A. N., Fay R. R. (1989). The ear as part of the octavolateralis system. In The Mechanosensory Lateral Line: Neurobiology and Evolution (ed. Coombs S., Görner P., Münz M.), pp. 633-651 Berlin: Springer-Verlag; [Google Scholar]
- Popper A. N. (1978). Scanning electron microscopic study of the otolithic organs in the bichir (Polypterus bichir) and shovel-nose sturgeon (Scaphirhynchus platorynchus). J. Comp. Neurol. 18, 117-128 [DOI] [PubMed] [Google Scholar]
- Popper A. N., Fay R. R., Platt C., Sand O. (2003). Sound detection mechanisms and capabilities of teleost fishes. In Sensory Processing of the Aquatic Environment (ed. Collin S. P., Marshall J. P.), pp. 3-23, New York: Springer Verlag; [Google Scholar]
- Rafinesque C. S. (1817). Addition to the observations on the sturgeons of North America. Amer. Mo. Mag. 1, 288 [Google Scholar]
- Retzius G. (1881). Das Gehörorgan der Wirbeltiere, Vol. 1 Stockholm: Samson and Wallin; [Google Scholar]
- Ruggero M. (1992). Physiology and coding of sound in the auditory nerve. In The Mammalian Auditory Pathway: Neurophysiology (ed. Popper A. N., Fay R. R.), pp. 34-93 New York: Springer-Verlag; [Google Scholar]
- Sand O., Michelsen A. (1978). Vibration measurements of the perch saccular otolith. J. Comp. Physiol. A 123, 85-89 [Google Scholar]
- Simmons D. D., Meenderink W. F., Vassilakis P. N. (2007). Anatomy, physiology and function of auditory end organs in the frog inner ear. In Hearing and Sound Communication in Amphibians (ed. Narins P. M., Feng A. S., Popper A. N., Fay R. R.), pp. 184-220 New York: Springer-Verlag; [Google Scholar]
- Steinacker A., Romero A. (1992). Voltage-gated potassium current resonance in the toadfish saccular hair cell. Brain Res. 574, 229-236 [DOI] [PubMed] [Google Scholar]
- Sugihara I., Furukawa T. (1989). Morphological and functional aspects of two different types of hair cells in the goldfish sacculus. J. Neurophysiol. 62, 1330-1343 [DOI] [PubMed] [Google Scholar]
- Tomchick S., Lu Z. (2006). Auditory physiology and anatomy of octavolateral efferent neurons in a teleost fish. J. Comp. Physiol. 192, 51-67 [DOI] [PubMed] [Google Scholar]
- Weeg M. S., Fay R. R., Bass A. H. (2002). Directional response and frequency tuning in saccular nerve fibers of a vocal fish, Porychthus notatus. J. Comp. Physiol. A 188, 631-641 [DOI] [PubMed] [Google Scholar]
- Wiley E. O. (1981). Phyologenetics – The Theory and Practice of Phylogenetic Systematics New York: John Wiley and Sons; [Google Scholar]