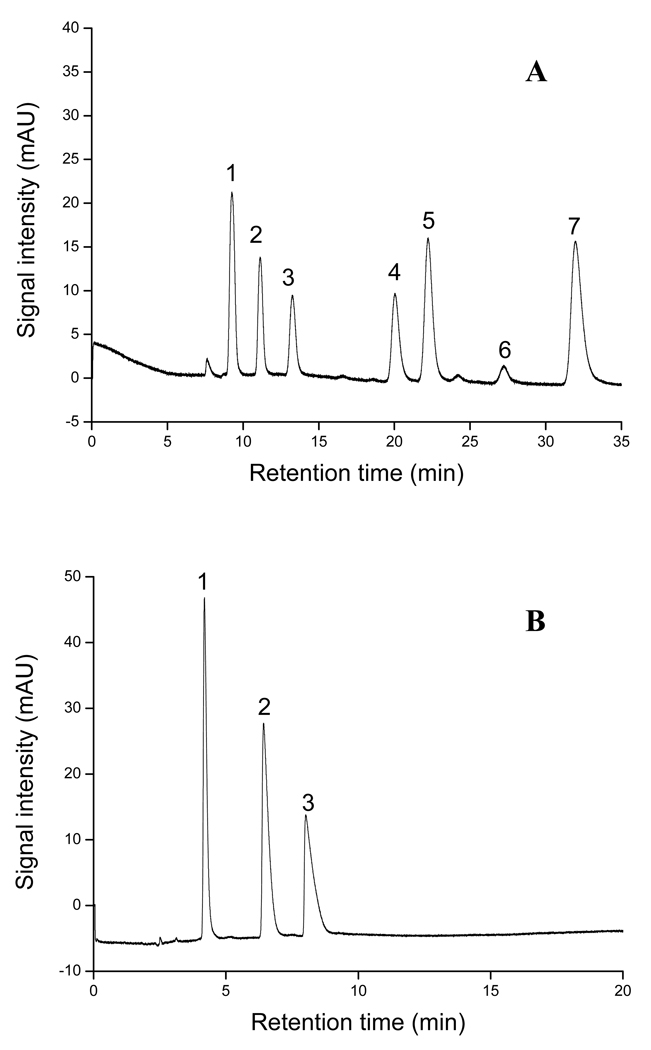
Figure 10.
Separation of (A) N-methylcarbamates (NMCs) pesticides and (B) phenylimidazole isomers obtained on the optimized monolithic column. Conditions: optimized monolithic column, 45cm total length (30cm effective length)×100 εm ID; (A) mobile phase, 5mM ammonium acetate, pH 6.5, at 30% (v/v) ACN; applied voltage, +25 kV; UV detection, 220 nm; electrokinetic injection, +5kV for 3 s. Analytes: 1, oxamy; 2, methomyl; 3, thiofanox sulfon; 4, aldicarb; 5, primicarb ; 6, propoxur; 7, aminocarb. Each analytes was injected at a concentration of 0.5 mg/mL prepared in 30% ACN/H2O. (B): mobile phase, 5mM phosphate buffer, pH 7.0, at 50% (v/v) ACN; applied voltage, +25 kV; UV detection, 214 nm; electrokinetic injection, +5kV for 3 s. Analytes: 1, 4-phenylimidazole; 2, 2-phenylimidazole; 3, 1-phenylimidazole.