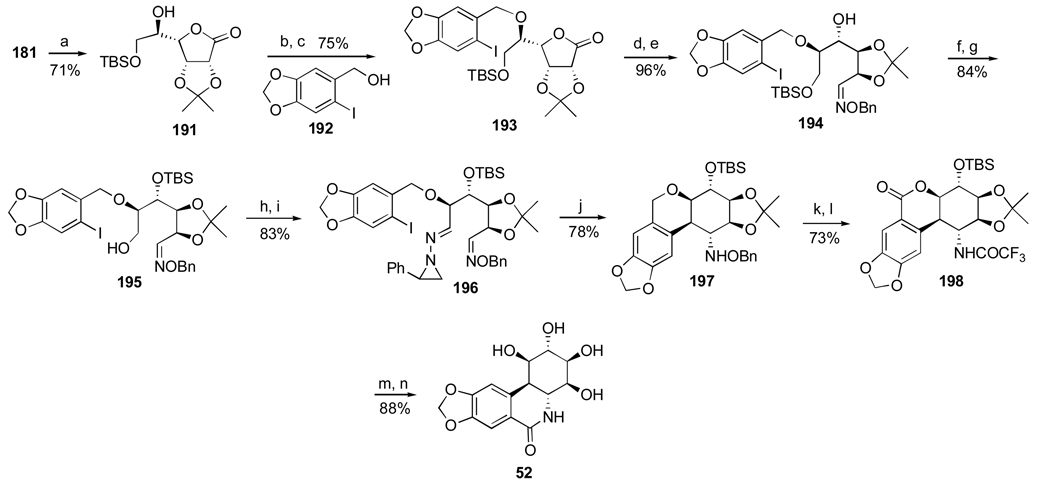
Scheme 17.a.
a Reagents and conditions: (a) TBSCl, imidazole; (b) 192, NaH, Cl3CCN, 0 °C; (c) TfOH, THF, 0 °C; (d) L-selectride, CH2Cl2, −78 °C; (e) HCl·H2NOBn, py; (f) TBSOTf, 2,6-lutidine, CH2Cl2, 0 °C; (g) HF·py, THF; (h) TPAP, NMO, 4Å MS; (i) 1-amino-2-phenylaziridine, EtOH, 0 °C; (j) Ph3SnH, AIBN, PhH, 78 °C; (k) SmI2, THF, then TFAA; (l) PCC, CH2Cl2, 55 °C; (m) BF3·Et2O, CH2Cl2; (n) K2CO3, MeOH.