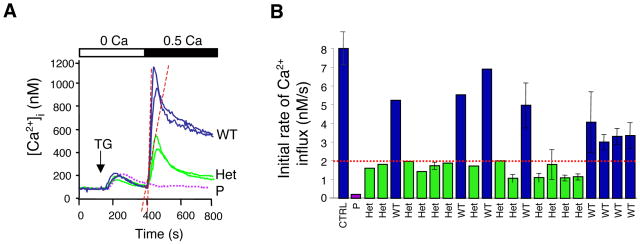
Figure 3. Phenotypic detection of putative heterozygous carriers.
Heterozygous carriers of an autosomal recessive mutation that impairs Ca2+ influx in immunodeficient patients were predicted based on in vitro analysis of SOCE in T cells from relatives of immunodeficient patients (see pedigree in Figure 4A). A, Ca2+ influx was measured in T cells stimulated with thapsigargin (TG) followed by readdition of lower than physiological extracellular Ca2+ (0.5 mM, black bar) to reveal a defect in Ca2+ influx. B, T cells from immunodeficient patients (purple trace in A and bar in B) lack SOCE, whereas T cells from relatives have normal (blue) or markedly reduced (green) Ca2+ influx, indicating that the latter, although healthy, may be heterozygous carriers of a recessive, disease-causing mutation. Not shown, The haplotypes of immunodeficient patients, predicted heterozygotes and predicted wild-type individuals from the same family were determined by genome-wide single nucleotide polymorphism (SNP) analysis and used to calculate multipoint parametric LOD scores in two independent analyses assuming an autosomal recessive and autosomal dominant mode of inheritance, respectively [17]. A 6.5 Mb interval on chromosome 12q24 containing the novel gene ORAI1 was identified to be linked to immunodeficiency disease with a LOD (log10 of the odds) score of 5.7. Identification of heterozygous carriers may be a useful tool for identification of gene defects in other rare diseases because it increases the number of haplotypes available for linkage analysis. DNA sequence analysis of ORAI1 revealed that all predicted carriers were indeed heterozygous for the ORAI1-R91W mutation. Figure modified from the version originally published in [17].