Abstract
Although research has established links between feelings of social isolation and inflammation, the direction of these effects is unclear. Based on the role that proinflammatory cytokines play in initiating “sickness behavior,” which includes symptoms such as social withdrawal, it is possible that inflammatory processes heighten feelings of ‘social disconnection.’ Here, we examined whether exposure to an inflammatory challenge increased self-reported feelings of social disconnection. In addition, because both inflammatory processes and feelings of social disconnection contribute to depressive symptoms, we also explored whether increases in feelings of social disconnection played a role in the link between inflammation and depressed mood. Participants were randomly assigned to either receive endotoxin, an inflammatory challenge, or placebo. Proinflammatory cytokines (IL-6, TNF-α) were collected at baseline and then hourly for six hours. Participants completed self-reports of sickness symptoms (“fatigue”), social disconnection (“I feel disconnected from others”), and depressed mood (“unhappy”) hourly. Results revealed that endotoxin led to significant increases (from baseline) in IL-6 and TNF-α levels as well as feelings of social disconnection and depressed mood. Moreover, controlling for increases in social disconnection eliminated the relationship between exposure to inflammatory challenge and depressed mood. This study demonstrates that inflammation can have social psychological consequences, which may play a role in cytokine-related depressive symptoms.
Keywords: proinflammatory cytokines, inflammation, social disconnection, depression, immune
Introduction
A large body of epidemiologic data indicates that feelings of social isolation are strongly associated with human physical health (Cacioppo and Hawkley, 2003; Seeman, 1996). Those who report feeling socially isolated have increased risk of all-cause mortality (Cacioppo and Hawkley, 2003; Seeman, 1996), as well as several specific infectious, neoplastic, cardiovascular, and inflammation-related diseases (Caspi et al., 2006; Cohen et al., 1997; Cole et al., 2003; Kroenke et al., 2006).
Although the biological basis for these links are not known, inflammatory processes may be involved. One study found that the leukocytes of socially isolated older adults (defined by feelings of loneliness) evidenced activation of inflammatory response genes along with increased activity of the nuclear factor (NF) κB pathway, a critical signal for the inflammatory cascade (Cole et al., 2007). Together, these cross-sectional data point toward an association between social isolation and inflammation. However, it is not known whether subjective feelings of social isolation—which we call ‘social disconnection’—activate inflammatory markers, whether inflammatory dynamics contribute to social disconnection, or some combination of both.
Research on neuroimmune signaling has shown that proinflammatory cytokines initiate “sickness behavior,” a coordinated motivational response that includes symptoms such as fatigue, anorexia, and social withdrawal, and is thought to facilitate recovery and recuperation from illness (Dantzer, 2001; Hart, 1988). Although a principal component of sickness behavior is social withdrawal, the psychological experience that accompanies these social changes has been largely overlooked. To the extent that there is a correlational relationship between feelings of social disconnection and inflammation, it is possible that cytokines may also increase feelings of social disconnection even in the absence of any overt changes in social behavior.
In addition, exploring the effect of inflammation on social disconnection may improve understanding of the emerging relationship between inflammation and depression (Miller et al., 2009) as both social disconnection and inflammation have been shown to play a role in depressive symptomatology. Thus, feelings of social disconnection (loneliness) have been shown to contribute to the development and maintenance of depression (Heinrich and Gullone, 2006), and cytokines have been shown to play a causal role in the onset of depressed or negative mood (Harrison et al., 2009; Reichenberg et al., 2001; Wright et al., 2005). As a result, it is possible that inflammatory-induced feelings of social disconnection may play a role in the link between inflammation and depressive symptoms. To date, however, these relationships have not been examined.
In this study, we examined the effect of endotoxin, an inflammatory challenge, vs. placebo on circulating levels of the proinflammatory cytokines—interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α)1—and on self-reported social disconnection and depressed mood. We hypothesized that endotoxin, compared to placebo, would lead to increases in social disconnection in addition to increases in depressed mood. In addition, we examined whether increases in social disconnection mediated the relationship between inflammation and depressed mood by examining whether controlling for increases in social disconnection eliminated the relationship between exposure to inflammatory challenge and increases in depressed mood.
Methods
Participants and Procedures
Thirty-nine healthy participants (20 female; mean age: 21.8 ± 3.4 years; range: 18–36 years) completed the study. Sample size was based on previous studies of experimentally-induced inflammatory challenge (Reichenberg et al., 2001). Participants were recruited from flyers posted at the UCLA Medical Center and advertisements posted in the campus newspaper. Prospective participants with the following conditions were excluded from participation through a structured telephone interview: claustrophobia, left-handedness, or metal in their body (relevant for a neuroimaging component that is reported separately, Eisenberger et al., 2009); chronic physical or mental health problems; history of allergies, autoimmune, or other severe chronic diseases; current use of prescription medications; or recent nightshift work or time zone shifts (> 3hrs).
After the telephone interview, if still eligible, participants completed an additional face-to-face screening interview to ensure eligibility for the study. During this session, participants completed the Structured Clinical Interview for DSM Disorders (SCID; First et al., 1996) and were asked a series of questions about their physical health and use of medications or drugs. In addition, height, weight, and vital signs were assessed, a urine sample was collected to examine drug use (marijuana, opiates, cocaine, amphetamines, methamphetamines), and blood was drawn to screen for abnormalities (e.g., complete blood cell count, chemistry panel, liver function tests) as well as pregnancy, if female. Any participant who: (1) had a BMI greater than 30, (2) reported physical health problems or medication use, (3) evidenced an Axis I psychiatric disorder based on the SCID assessment, (4) showed evidence of drug use from a positive urine test, (5) had a positive pregnancy test, or (6) showed abnormalities on the screening laboratory tests were ineligible for the study. The final sample was 39% European-American, 18% Asian, 18% Hispanic, 7% African-American, and 18% “other.”
The study was conducted between January and November 2007 at the UCLA General Clinical Research Center (GCRC) using a randomized, double-blind, placebo-controlled design. Upon arrival to the GCRC, a nurse, who was blind to condition, inserted a catheter with a heparin lock into the dominant forearm (right) for hourly blood draws and one into the non-dominant forearm (left) for a continuous saline flush and for drug administration. Ninety minutes after arrival at the GCRC, each participant was randomly assigned to receive either endotoxin (0.8 ng/kg of body weight; n=23, 12 females) or placebo (same volume of 0.9% saline; n=16, 8 females), which was administered by the nurse as an intravenous bolus. The endotoxin used was derived from Escherichia coli (E. coli group O:113, provided by the National Institutes of Health Clinical Center) and has been shown to be safe for studies of experimental inflammation in humans (Andreasen et al., 2008; Suffredini et al., 1999; Suffredini and O’Grady, 1999). Random assignment was determined by a consultant who was not involved in running participants and was kept by the UCLA Pharmacy to ensure proper drug preparation for each participant. The random allocation sequence was determined through the use of a random number generator with consideration for the inclusion of equal numbers of males and females in each group. No significant differences in age, years of education, or body weight were found between the two groups (p’s>.22).
Throughout the study, vital signs (temperature, pulse, blood pressure) were assessed every half hour and blood draws (to assess IL-6 and TNF-α) were collected at baseline and then approximately every hour for the next six hours. Participants also completed hourly measures of sickness symptoms (e.g., fatigue), feelings of social disconnection, and depressed mood. For safety reasons, the study physician (M.R.I.) was aware of each participant’s group assignment and was on call during each of the experimental sessions, but did not take part in the testing procedures. Participants were discharged from the GCRC following the last blood draw upon approval from the study’s physician; approval was granted if self-reported physical and psychological symptoms returned to baseline levels. All participants left the study feeling as good as they did when they started, and there were no adverse events. At the end of the study, participants were thanked, debriefed, and paid for their participation ($200). Experimental procedures were approved by the UCLA Human Subjects Protection Committee.
Behavioral Assessments
Self-reported sickness symptoms
Physical sickness symptoms (muscle pain, shivering, nausea, breathing difficulties, fatigue) were assessed at baseline and then hourly following drug/placebo adminstration for six hours. Participants rated the extent to which they felt each symptom on a scale from 0 (no symptoms) to 4 (very severe symptoms).
Feelings of social disconnection
Feelings of social disconnection were assessed hourly. Participants rated the extent to which they were feeling the “following feelings right now” on a 5-point Likert scale (1—not at all, to 5—very much so): (1) “I feel like being around other people,” (2) “I feel like being alone,” (3) “I feel overly sensitive around others (e.g., my feelings are easily hurt),” (4) “I feel connected to others,” and (5) “I feel disconnected from others.” Items 1 and 4 were reverse-coded, and scores were averaged at each time point to create a measure of self-reported social disconnection. The reliability of the scale (assessed at the time of peak response) was high (α=.84).
Depressed mood
Depressed mood was assessed hourly, using an abbreviated version of the Profile of Mood States (McNair et al., 1971). Participants rated the extent to which they felt: “unhappy,” “blue,” “lonely,” “gloomy,” and “worthless” on a scale from 0 (not at all) to 4 (extremely). Depressed mood was calculated by averaging scores from each of these items at each timepoint. The reliability of the scale (assessed at the time of peak response) was good (α=.68).
Plasma Levels of Cytokines
Plasma blood samples were collected in pre-chilled tubes containing sodium ethylenediaminetetraacetic acid and aprotinin. Tubes were immediately centrifuged at 4°C, plasma was harvested into multiple aliquots, and then frozen in a −70°C freezer. Plasma levels of IL-6 and TNFα were quantified by means of high sensitivity enzyme-linked immunosorbent assays (Quantikine HS Human IL-6, Quantikine HS Human TNF-α, R & D Systems, Minneapolis, MN). All assays were performed according to the manufacturer’s protocols, with reported intra-assay and inter-assay coefficients of variation less than 11%. For IL-6 assays, the lower limit of quantitation was 0.2 pg/ml; all samples were assayed in duplicate. For TNF-α assays, the lower limit of quantitation was 0.5 pg/ml; repeated measures on each individual were assayed in single wells.
In order to minimize the potential effects of inter-assay variation, all samples from the same subject were initially assayed in the same assay run; samples with cytokine concentrations exceeding the upper limit of quantitation were diluted as directed in the assay protocol and repeated. IL-6 levels were evaluated at all time points for every subject. TNF-α levels were determined at all time points for subjects in the endotoxin condition. TNF-α levels at all time points were examined for four subjects in the placebo condition, and were observed to vary less than or equal to 0.3 pg/ml within each subject. In light of the stable values observed, all remaining subjects in the placebo condition were assayed for TNF-α at baseline and two hours post-injection, which corresponded to the peak of TNF-α seen in endotoxin-exposed subjects.
Statistical Analyses
To assess between-group differences in the effect of endotoxin vs. placebo on cytokine levels, objective physical symptoms (e.g., blood pressure), self-reported sickness symptoms, and feelings of social disconnection and depressed mood, we used a standard statistical software program (SPSS 16.0) to conduct repeated-measure analyses of variance. These analyses tested time (baseline vs. each subsequent time point) by condition (endotoxin vs. placebo) interactions, as has been done previously (Reichenberg et al., 2001). Because IL-6 levels were not normally distributed at any of the time points, each value was log-transformed. It should be noted that for TNF-α, we could only examine time by condition interactions comparing baseline with T2, as only a small subsample of the placebo subjects’ plasma samples were assayed at the other time points (T1, T3-6; n=4).
As an exploratory analysis, we also examined whether increases in cytokine responses to endotoxin correlated with increases in self-reported feelings of social disconnection. To do this, we examined whether subjects in the endotoxin group showed significant correlations between increases in proinflammatory cytokine levels (difference scores comparing baseline to T2) and increases in self-reported social disconnection (difference scores comparing baseline to T2).
Finally, because of the well-known sex differences in the prevalence of inflammatory disorders and depression (Nolen-Hoeksema, 2001; Whitacre et al., 1999), we also examined sex differences in the effects of endotoxin. To do this, we conducted repeated-measures analyses of variance to examine time (baseline vs. each subsequent time point) by condition by sex interactions on all outcome variables. Significant interactions were then explored to examine the direction of the effects.
Results
Physiological Responses to Endotoxin
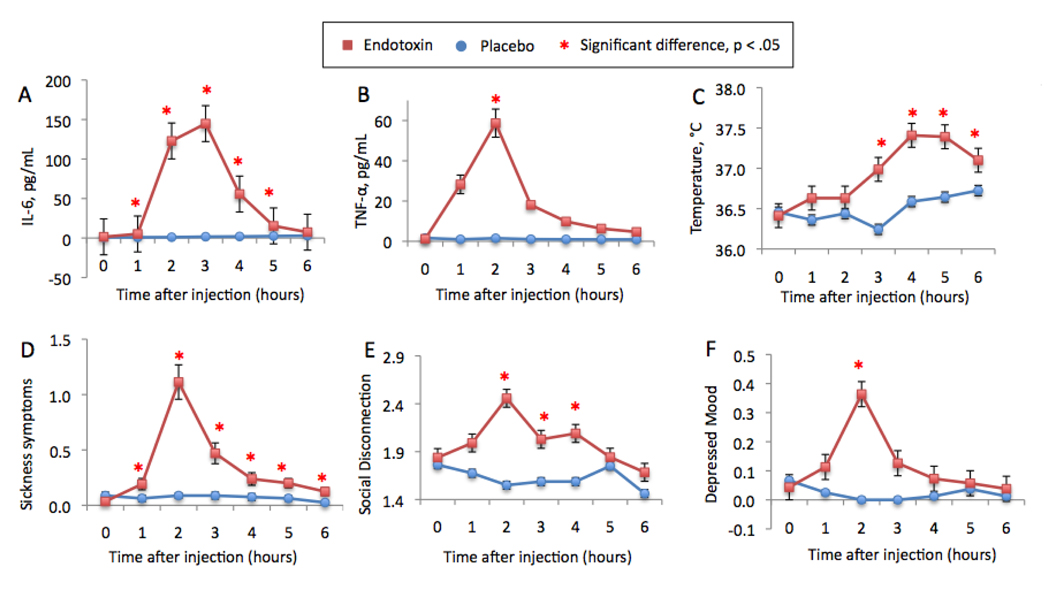
As reported previously (Eisenberger et al., 2009), endotoxin induced a significant increase in IL-6 levels from one to five hours post-injection (reflected by significant time by condition interactions at each time point compared to baseline: T1 (one hour post-injection) through T5 (five hours post-injection): F(1,37)=23.19, 144.14, 177.11, 63.67, 11.36, p’s<.005; Figure 1a, previously displayed in Eisenberger et al., 2009). Endotoxin also induced a significant increase in TNF-α levels from baseline to two hours post-injection (F(1,37)=46.79, p<.001 (Figure 1b). Because we did not assay TNF-α levels for the placebo group at the other time points, we could not assess other time by condition interactions. However, paired-samples t-tests revealed that, among those exposed to endotoxin, TNF-α levels were significantly higher at all post-injection time points compared to baseline (all p’s<.001). Regarding physical symptoms, endotoxin led to significant increases in body temperature (T3-T6: F(1,37)=14.18, 17.14, 17.93, 5.70, p’s<.05; Figure 1c) and pulse (T3-T6: F(1,37)=48.43, 24.01, 23.74, 14.59, p’s<.001), but not to changes in blood pressure (p’s>.10). In addition, endotoxin induced a significant increase in sickness symptoms at all measured time points (F(1,37)=9.08, 32.37, 13.60, 8.54, 11.65, 8.01, p’s<.01; Figure 1d). There were no sex differences in any of these effects (p’s>.16).
Figure 1.
Changes over time in the endotoxin and placebo groups in: (A) plasma levels of IL-6 (raw values that have not been log-transformed; previously reported in Eisenberger et al., 2009), (B) plasma levels of TNF-α (note that values for T1 and T3-T6 do not include data for all placebo subjects), (C) oral temperature, (D) self-reported sickness symptoms, (E) self-reported feelings of social disconnection, and (F) self-reported depressed mood (previously reported in Eisenberger et al., 2009). Time points with asterisks indicate significant time (baseline vs. each subsequent time point) by condition interactions.
Social and Affective Responses to Endotoxin
Feelings of social disconnection
Confirming the notion that proinflammatory cytokines can increase feelings of social disconnection, we found that endotoxin (vs. placebo) led to significant increases (from baseline) in self-reported social disconnection at two (F(1,35)=16.41, p<.001), three (F(1,35)=4.01, p=.05), and four (F(1,34)=4.54, p<.05) hours post-injection (Figure 1e).2 Controlling for increases in each type of sickness symptom (focusing on the time of peak response—T2) did not change this relationship (p’s<.05), suggesting that increases in social disconnection were not solely due to increasing sickness symptoms.
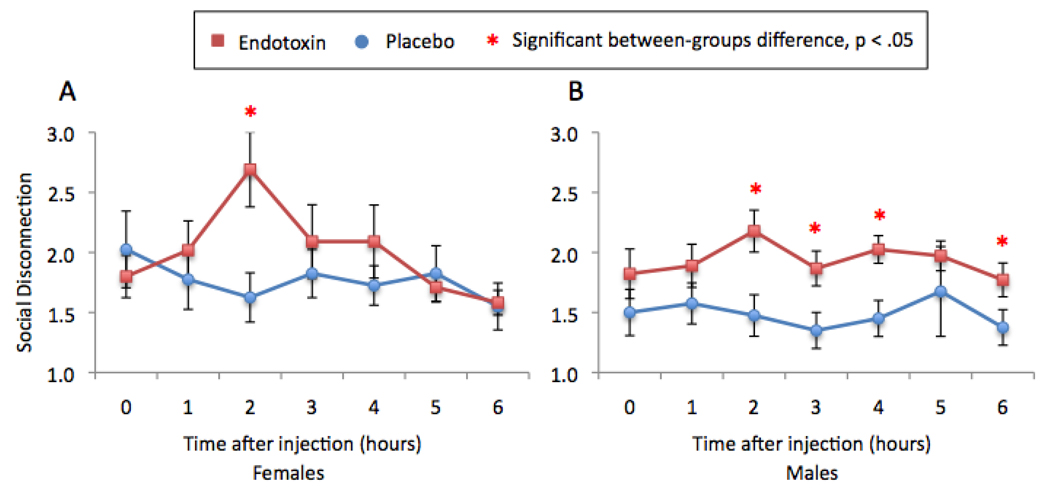
In addition, there was a significant time (baseline vs. T2) by condition by sex interaction (F(1,33)=6.02, p < .05), such that females showed a greater increase in social disconnection from baseline to two hours post-injection (F(1,17)=20.93, p<.001) than males (F(1,16)=1.75, p=.21; Figure 2). However, upon closer inspection, it appeared that, when focusing on between-group differences at the time of peak response, endotoxin led to significantly higher levels of social disconnection than placebo in both males and females (males: F(1,17)=9.55, p<.01; females: F(1,18)=5.26, p<.05). Thus, even though females showed a larger increase in feelings of social disconnection than males (from baseline to T2), both males and females showed significant between-group differences in feelings of social disconnection at two hours post-injection. Moreover, for males, endotoxin (vs. placebo) led to significantly higher levels of social disconnection at three (F(1,17)=87.42, p < .05), four (F(1,16)=10.86, p<.01), and six hours (F(1,15)=4.59, p=.05) post-injection as well (see Figure 2), suggesting that the effects of endotoxin on feelings of social disconnection in males may be more enduring.
Figure 2.
Changes over time in the endotoxin and placebo groups in self-reported feelings of social disconnection for (A) females and (B) males. Time points with asterisks indicate significant between-group (endotoxin vs. placebo) differences.
Additional exploratory analyses were performed to evaluate associations between increases in IL-6 and TNF-α (from baseline to T2) and increases in self-reported social disconnection (from baseline to T2) among subjects in the endotoxin group. There was one multivariate outlier (determined by Cook’s Distance residuals) who was removed from these analyses. Analyses revealed that greater increases in IL-6 and TNF-α were associated with greater increases in feelings of social disconnection (IL-6: r=.35, p=.13; TNF-α: r=.48, p<.05). When examining males alone, greater increases in TNF-α, but not IL-6, were marginally significantly associated with greater increases in social disconnection (r=.61, p=.08). Finally, for females, greater increases in IL-6 and TNF-alpha levels were not significantly associated with greater increases in social disconnection; however, the effect sizes were comparable to those observed for the full sample (IL-6: r=.36, p=.27; TNF-α: r=.40, p=.23), and thus could reach statistical significance with a larger sample. These results should be interpreted with caution due to the small sample sizes (18 endotoxin subjects with complete data: 11 females, 7 males).
Depressed mood
As reported previously (Eisenberger et al., 2009), endotoxin (vs. placebo) led to a significant increase (baseline to T2) in self-reported depressed mood (F(1,35)=8.13, p<.01; Figure 1f, previously displayed in Eisenberger et al., 2009)3, and there were no sex differences in this effect (p>.26). In addition, the effect of endotoxin on depressed mood was not substantially altered by controlling for each type of sickness symptom (p’s<.11), with the exception of fatigue. Not surprisingly, given conceptual overlaps between fatigue and depressed mood, controlling for increases in fatigue led to a non-significant time by condition interaction on depressed mood (F(1,34)=.10, ns).
A Role for Social Disconnection in Endotoxin-Induced Depressed Mood
Finally, we explored whether increases in feelings of social disconnection mediated the relationship between condition and increases in depressed mood (baseline to T2). Although we cannot assess temporal mediation (e.g., whether condition altered feelings of social disconnection which then led to depressed mood), as both feelings of social disconnection and depressed mood peaked at the same time point, we can examine whether self-reported increases in feelings of social disconnection carried the influence of condition (endotoxin vs. placebo) on increases in depressed mood. Interestingly, a mediation analysis revealed that increases in feelings of social disconnection were a significant mediator of the relationship between condition and increases in depressed mood (Sobel test: Z=3.21, p<.005). Thus, when controlling for increases in social disconnection, the relationship between condition and increases in depressed mood was no longer significant (F(1,32)=.02, ns). Controlling for increases in depressed mood, however, did not eliminate the relationship between condition and increases in social disconnection (F(1,32)=7.84, p<.01).4 Thus, feelings of social disconnection may play a role in inflammatory-induced depressed mood.
Discussion
Findings from this study extend previous work on endotoxin-induced depressed or negative mood (Harrison et al., 2009; Reichenberg et al., 2001; Wright et al., 2005) by demonstrating that an inflammatory challenge can have social consequences as well. Thus, in addition to endotoxin (vs. placebo) leading to significant increases in depressed mood, it also led to significant increases in feelings of social disconnection. Moreover, increases in social disconnection mediated the relationship between inflammatory activity and depressed mood, such that controlling for these social psychological changes eliminated the relationship between exposure to inflammatory challenge and depressed mood. Thus, inflammatory activity may have important social psychological consequences, which could play a role in the link between inflammation and depression. Future research will be needed to examine the temporal sequence of these effects as, in the current study, both self-reported feelings of social disconnection and depressed mood peaked at the same time point. Thus, it would be interesting to investigate whether feelings of social disconnection play a temporal role in the longer-term links between inflammatory activity and depressive symptoms more generally.
These results dovetail nicely with several previous findings. First, although the effect of inflammation on social experience has not been examined in humans, the notion that inflammation contributes to social disconnection fits with animal studies, demonstrating that social withdrawal is an important component of inflammation-induced sickness behavior (Hart, 1988). Furthermore, the notion that feelings of social disconnection play a role in cytokine-induced depressed mood aligns with research that has separately shown that inflammation (Miller et al., 2009), as well as feelings of social disconnection (Heinrich and Gullone, 2006), contribute to depressive symptomatology. Indeed, previous work in rodents has shown that inflammatory-induced social withdrawal was reversed by antidepressant treatment (Yirmiya, 1996), suggesting a mechanistic relatedness between inflammation-induced social changes and mood changes. Nonetheless, although these links between inflammation, social disconnection, and depressed mood are suggestive, it should be noted that the current research only examined individuals who were free of clinical depression. Thus, additional research will be needed to examine how these effects unfold in depressed individuals as well as how inflammation-induced social disconnection may or may not play a role in clinical forms of depression.
In addition, further work will be needed to more carefully examine the specific social psychological changes that occur as a function of inflammation using more well-validated measures. The questionnaire that was used to assess feelings of social disconnection in the current study included items that both reflected a desire to socially withdraw (e.g., “I feel like being alone”) as well as items that reflected a feeling of being socially isolated or disconnected (e.g., “I feel disconnected from others). Although both types of items were altered as a function of endotoxin, additional work is needed to determine the specific types of social cognitions that are altered by inflammatory processes. For instance, it would be interesting to examine whether inflammatory activity also decreases the sense that one is socially supported, as a perceived lack of social support is a risk factor for both endogenous and cytokine-associated forms of depression (Capuron et al., 2004; Heinrich and Gullone, 2006).
In sum, the findings presented here provide the first evidence that an experimental inflammatory challenge can increase feelings of social disconnection and that these changes may play a role in depressed mood. Future research that investigates the social consequences of inflammation is needed. Understanding how inflammation alters social experience may shed new light on the emerging links between inflammation and depression.
Acknowledgements
We would like to thank the staff and support of the UCLA General Clinical Research Center as well as Anthony Suffredini, M.D. and George Grimes, R.P. at the National Institutes of Health, Warren Grant Magnuson Clinical Center, for providing the standard reference endotoxin and Thanh Luu and Elizabeth Breen for performing the cytokine assays. This research was funded by a NARSAD Young Investigator Award, a Dana Foundation grant, a UCLA Faculty Senate Grant, and a postdoctoral research fellowship (T32-MH19925) to N.I.E. In addition, the authors acknowledge the additional support provided by grants HL-079955, AG-026364, CA-10014152, CA-116778, P30-AG028748, M01-RR00865, the UCLA Cousins Center at the Semel Institute for Neurosciences, the UCLA Claude D. Pepper Older Americans Independence Center Inflammatory Biology Core, and the General Clinical Research Centers Program (M01-RR00865).
Footnotes
It should be noted that endotoxin has been shown to lead to a coordinated pattern of increases across several different proinflammatory cytokines (e.g., IL-6, TNF-α, IL-1 receptor antagonist) and thus we are not suggesting that the effects of endotoxin are primarily or uniquely related to IL-6 or TNF-α levels. In the current study, however, we examined IL-6 and TNF-α levels, as these markers of inflammation have previously been shown to correlate with measures of depression (Miller et al., 2009) and to map along with other markers of inflammation (e.g., IL-1 receptor antagonist) in response to inflammatory challenge (Reichenberg et al., 2001).
Secondary analyses, focusing on the time of peak response (two hours post-injection), revealed significant time by condition interactions for each social disconnection item on its own (all p’s < .05), with the exception of the item “I feel overly sensitive around others” (F(1,35)=.57, ns).
Because the depressed mood scale included an item assessing “lonely” feelings—which overlaps conceptually with social disconnection—we also reran all the reported analyses (involving depressed mood) without the lonely item included in the scale. None of the results were altered by this change, and thus we report the depressed mood subscale that included the “lonely” item in order to be consistent with prior work (Eisenberger et al., 2009).
Again, none of these results were altered by removing the item assessing “lonely” feelings from the depressed mood scale.
The authors have no financial gain related to the outcome of this research, and there are no potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andreasen AS, Krabbe KS, Krogh-Madsen R, Taudorf S, Pedersen BK, Møller K. Human endotoxemia as a model of systemic inflammation. Current Medicinal Chemistry. 2008;15:1697–1705. doi: 10.2174/092986708784872393. [DOI] [PubMed] [Google Scholar]
- Cacioppo JT, Hawkley LC. Social isolation and health, with an emphasis on underlying mechanisms. Perspectives in Biology and Medicine. 2003;46:S39–S52. [PubMed] [Google Scholar]
- Capuron L, Ravaud A, Miller AH, Dantzer R. Baseline mood and psychosocial characteristics of patients developing depressive symptoms during interleukin-2 and/or interferon-alpha cancer therapy. Brain, Behavior, and Immunity. 2004;18:205–213. doi: 10.1016/j.bbi.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Caspi A, Harrington BS, Moffitt TE, Milne BJ, Poulton R. Socially isolated children 20 years later. Archives of pediatrics and Adolescent Medicine. 2006;160:805–811. doi: 10.1001/archpedi.160.8.805. [DOI] [PubMed] [Google Scholar]
- Cohen S, Doyle WJ, Skoner DP, Rabin BS, Gwaltney JM., Jr Social ties and susceptibility to the common cold. Journal of the American Medical Association. 1997;277:1940–1944. [PubMed] [Google Scholar]
- Cole SW, Hawkley LC, Arevalo JM, Sung CY, Rose RM, Cacioppo JT. Social regulation of gene expression in human leukocytes. Genome Biology. 2007;8:R189. doi: 10.1186/gb-2007-8-9-r189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole SW, Kemeny ME, Fahey JL, Zack JA, Naliboff BD. Psychological risk factors for HIV pathogenesis: Mediation by the automatic nervous system. BiologicalPsychiatry. 2003;54:1444–1456. doi: 10.1016/s0006-3223(02)01888-7. [DOI] [PubMed] [Google Scholar]
- Dantzer R. Cytokine-induced sickness behavior: Mechanisms and implications. Annals of the New York Academy of Sciences. 2001;933:222–234. doi: 10.1111/j.1749-6632.2001.tb05827.x. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI, Inagaki TK, Rameson LT, Mashal NM, Irwin MR. An fMRI study of cytokine-induced depressed mood and social pain: The role of sex differences. NeuroImage. 2009;47:881–890. doi: 10.1016/j.neuroimage.2009.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JB. Structured clinical interview for DSM-IV Axis I disorders, Patient Edition, Version 2.0. New York: State Psychiatric Institute; 1996. [Google Scholar]
- Harrison NA, Brydon L, Walker C, Gray MA, Steptoe A, Dolan RJ, Critchley HD. Neural origins of human sickness in interoceptive responses to inflammation. Biological Psychiatry. 2009;66:415–422. doi: 10.1016/j.biopsych.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart BL. Biological basis of the behavior of sick animals. Neuroscience & Biobehavioral Review. 1988;12:123–137. doi: 10.1016/s0149-7634(88)80004-6. [DOI] [PubMed] [Google Scholar]
- Heinrich LM, Gullone E. The clinical significance of loneliness: A literature review. Clinical Psychology Review. 2006;26:695–718. doi: 10.1016/j.cpr.2006.04.002. [DOI] [PubMed] [Google Scholar]
- Kroenke CH, Kubzansky LD, Scherhammer ES, Holmes MD, Kawachi I. Social networks, social support, and survival after breast cancer diagnosis. Journal of Clinical Oncology. 2006;24:1105–1111. doi: 10.1200/JCO.2005.04.2846. [DOI] [PubMed] [Google Scholar]
- McNair DM, Lorr M, Droppleman LF. Manual for the Profile of Mood States. San Diego, CA: Education and Industrial Testing Service; 1971. [Google Scholar]
- Miller AH, Maletic V, Raison CL. Inflammation and its discontents: The role of cytokines in the pathophysiology of major depression. Biological Psychiatry. 2009;65:732–741. doi: 10.1016/j.biopsych.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolen-Hoeksema S. Gender differences in depression. Current Directions in Psychological Science. 2001;10:173–176. [Google Scholar]
- Reichenberg A, Yirmiya R, Schuld A, Kraus T, Haack M, Morag A, Pollmächer T. Cytokine-associated emotional and cognitive disturbances in humans. Archives of General Psychiatry. 2001;58:445–452. doi: 10.1001/archpsyc.58.5.445. [DOI] [PubMed] [Google Scholar]
- Seeman TE. Social ties and health: The benefits of social integration. Annals of Epidemiology. 1996;6:442–451. doi: 10.1016/s1047-2797(96)00095-6. [DOI] [PubMed] [Google Scholar]
- Suffredini AF, Fantuzzi G, Badolato R, Oppenheim J, O’Grady N. New insights into the biology of the acute phase response. Journal of Clinical Immunology. 1999;19:203–214. doi: 10.1023/a:1020563913045. [DOI] [PubMed] [Google Scholar]
- Suffredini AF, O’Grady NP. Pathophysiologic responses to endotoxin in humans. In: Braude H, Opal SM, Vogel SN, Morrison DC, editors. Endotoxin in Health and Disease. 1st Edition. New York: Marcel Dekker; 1999. pp. 817–830. [Google Scholar]
- Whitacre CC, Reingold SC, O’Looney PA. A gender gap in autoimmunity. Science. 1999;283:1277–1278. doi: 10.1126/science.283.5406.1277. [DOI] [PubMed] [Google Scholar]
- Wright CE, Strike PC, Brydon L, Steptoe A. Acute inflammation and negative mood: Mediation by cytokine activation. Brain, Behavior, & Immunity. 2005;19:345–350. doi: 10.1016/j.bbi.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Yirmiya R. Endotoxin produces a depressive-like episode in rats. Brain Research. 1996;711:163–174. doi: 10.1016/0006-8993(95)01415-2. [DOI] [PubMed] [Google Scholar]