Figure 1.
Selection of an Improved Acetyl-Lysyl-tRNA Synthetase/tRNACUA Pair for the Incorporation of Acetyl-Lysine in Recombinant Proteins
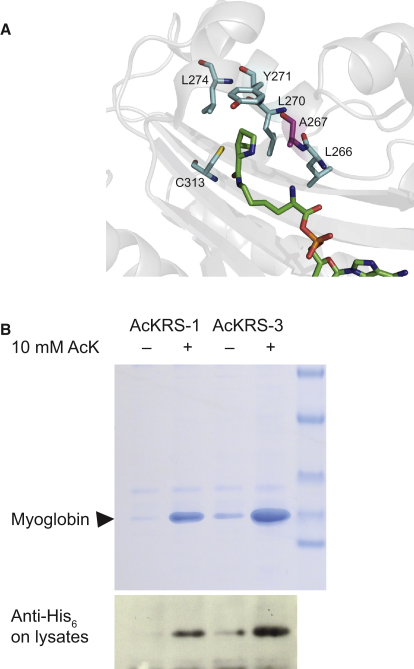
(A) The active site of M. mazei PylRS bound to pyrrolysine (figure created using PyMOL [http://www.pymol.org] and PDB file 2Q7H). The residues mutated relative to the wild-type sequence are shown as sticks. Residues in cyan are mutated in the progenitor AcKRS-1 and were randomized again in the new library; A267 (magenta) was only included in the new library.
(B) Characterization of a more efficient acetyl-lysyl-tRNA synthetase/tRNACUA pair. Myoglobin-His6 was expressed in E. coli DH10B from pMyo4TAG PylT (Neumann et al., 2008) (containing a hexa-histidine-tagged myoglobin gene with an amber codon at position 4 and the gene encoding MbtRNACUA) in the presence or absence of 10 mM acetyl-lysine using either pBK AcKRS-1 or pBK AcKRS-3. The proteins were purified by Ni2+ chromatography and analyzed by 4%–12% SDS-PAGE or detected in total lysates by western blot with an anti-His6 antibody.