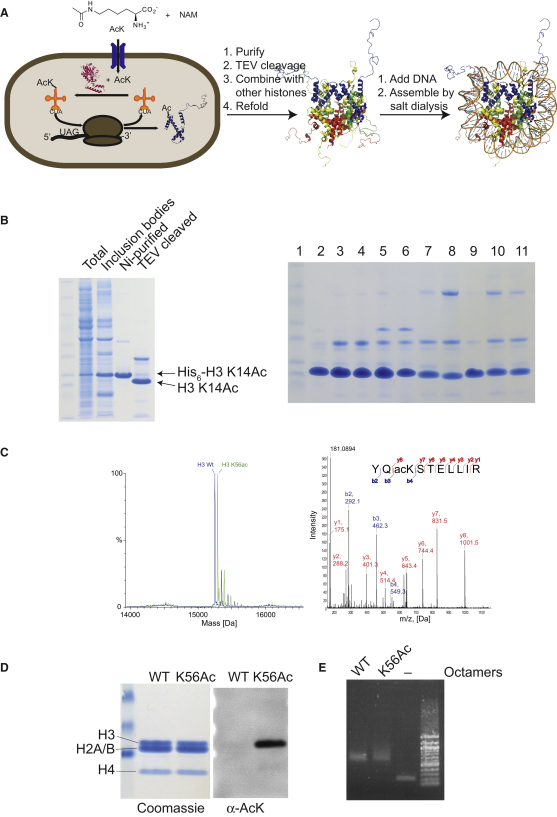
Figure 2.
The Expression and Purification of Site-Specifically Acetylated Histones and the Assembly of Histone Octamers and Nucleosomes
(A) Schematic illustration showing the recombinant expression of site-specifically acetylated recombinant histones in E. coli and their reconstitution into histone octamers and nucleosomes.
(B) (Left) The expression, purification, and TEV cleavage of histone H3 K14Ac is followed by SDS PAGE. (Right) Purified and TEV cleaved site-specifically acetylated histones. (1) molecular weight marker, (2) H3 WT, (3) H3 K14Ac, (4) H3 K23Ac, (5) H3 K27Ac, (6) H3 K56Ac, (7) H2A WT, (8) H2A K9Ac, (9) H2B WT, (10) H2B K5Ac, and (11) H2B K20Ac.
(C) Electrospray ionization mass spectrometry demonstrates that the protein is homogeneously acetylated, and MS/MS of tryptic peptides identifies the site of acetylation at lysine 56, as genetically encoded. The smaller peak to the right of the main peak is 98 Da heavier and corresponds to a phosphate from buffer associated with the histone.
(D) H3 K56Ac assembles into octamers with comparable efficiency to unmodified H3. Denaturing (4%–12%) SDS-PAGE of assembled octamers. The acetylation of H3 in the octamer is confirmed by western blot with an anti-acetyl-lysine antibody.
(E) Reconstitution of unmodified octamers and octamers bearing H3 K56Ac into nucleosomes with 197bp 601 DNA. Nucleosomes and free DNA were resolved by 0.8% agarose gel and stained with ethidium bromide.