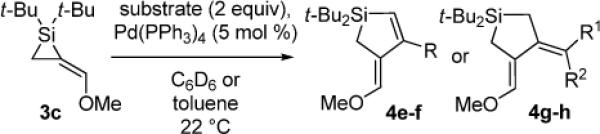
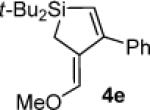
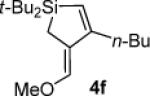
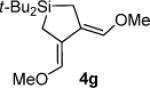
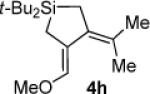
Table 3.
Pd(0)-Catalyzed Carbon–Carbon Bond Insertion with 3c to Yield Si–C(sp2) Bond Cleavage Products
As determined by 1H NMR spectroscopic analysis relative to an internal standard (PhSiMe3). Isolated yields are shown in parentheses.
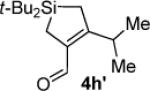
Hydrolysis gave (α,β-unsaturated aldehyde 4h′ when subjected to chromatography.