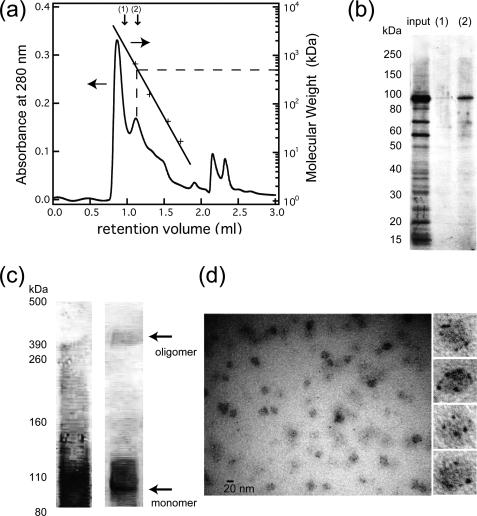
FIGURE 2.
Oligomerization analysis of purified His-rTRPV4. a, shown is a size-exclusion chromatogram of proteins in the detergent-solubilized state after affinity purification. The retention volume of the molecular mass standards is shown by crosses for thyroglobulin (670 kDa), γ-globulin (158 kDa), ovalbumin (44 kDa), and myoglobin (17 kDa). The molecular mass of His-rTRPV4 (including detergent molecules) was estimated as 500 kDa. b, SDS-PAGE analysis of the input, fraction 1, and fraction 2 indicated in the size-exclusion chromatogram was assessed by Coomassie staining. c, chemical cross-linking analysis of purified His-rTRPV4 was assessed by Coomassie staining (left) and a Western blot using anti-His-tag antibody (right). The molecular weight of a cross-linked band above 390 kDa is consistent with a tetramer of His-rTRPV4 subunits (103 kDa). d, shown is a general view of a Nanogold-labeled and NanoVan-stained His-rTRPV4 and an enlarged view of particles having multiple Nanogolds.