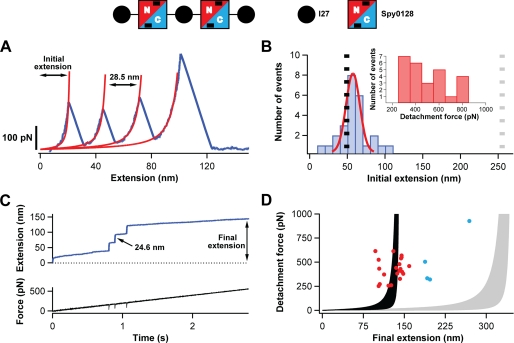
FIGURE 1.
Study of the extensibility of Spy0128 domains. A schematic of the protein construct used to probe the mechanical response of Spy0128 and (I27-Spy0128)2-I27 is shown. Two Spy0128 molecules (squares) were inserted between I27 domains (black circles). Spy0128 is composed of two domains (N terminus, red; C terminus, blue). Intramolecular isopeptide bonds are represented by black bars. A, typical force-extension trace (blue) showing three peaks corresponding to the unfolding of the three I27 domains in the protein construct. In red, fits to the worm-like chain model are shown. Even though the Spy0128 domains must have been subject to force, no unfolding peaks corresponding to Spy0128 are identified. B, histogram of the initial extension for traces with three I27 domains unfolding events (n = 26). The expected initial extensions if the two Spy0128 behaved as random coils (gray dotted line) or as mechanically stable domains (black dotted line) are shown. A Gaussian fit to the histogram is shown in red. Inset, histogram showing the detachment force in the force-extension experiments. C, top, typical force-ramp trace characterized by three steps marking the unfolding of the I27 modules. No steps corresponding to the Spy0128 domains were observed. Bottom, time course of the force experienced by the polyprotein. The unfolding events appear as negative spikes, reflecting the time response of the feedback system (33). D, scatter plot showing the final extension in the force-ramp experiments versus the detachment force (n = 25). The shaded regions represent the worm-like chain model of polymer elasticity (persistence lengths between 0.2 and 2.3 nm) if the Spy0128 domains remain folded (black) or, in contrast, unfold during the force-ramp protocol (gray) (28). The experimental points not following the general trend are colored in blue.