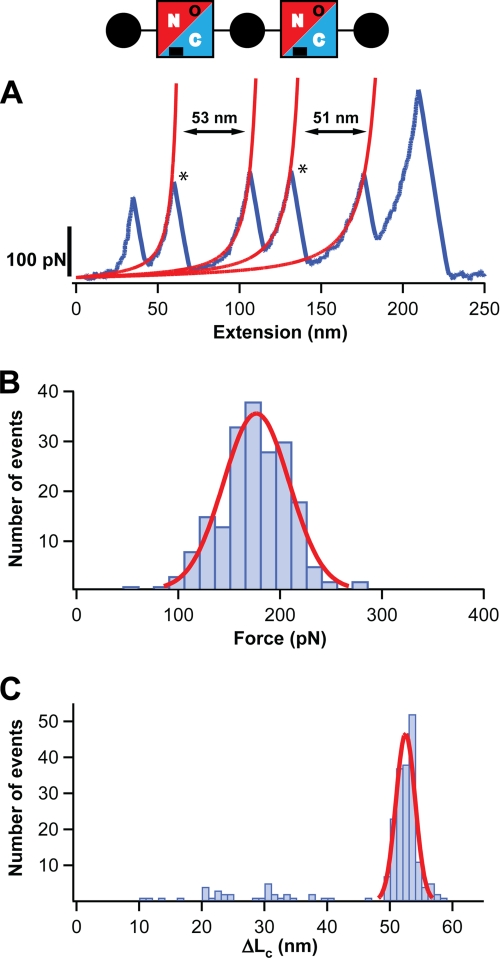
FIGURE 2.
The N-terminal domain of Spy0128 E117A extends upon the application of force. A diagram of the protein construct employed, (I27-Spy0128-E117A)2-I27, is shown (see also legend to Fig. 1). The mutation E117A abolishes the formation of the isopeptide bond at the N-terminal domain of Spy0128 (in the diagram, this is denoted by an open circle). A, typical force-extension trace for (I27-Spy0128-E117A)2-I27 is shown in blue. The peaks marking the unfolding of the Spy0128 N-terminal domains in the polyprotein are labeled with an asterisk. The increment in contour length after the Spy0128 unfolding events is calculated from the worm-like chain fits shown in red. B, histogram of the unfolding forces for the N-terminal domain in Spy0128 E117A (n = 197). The solid line corresponds to a Gaussian fit of the experimental data. C, histogram of the ΔLc associated with the unfolding of the N-terminal domain in Spy0128 E117A (n = 214). A Gaussian fit for the data above 45 nm is shown (solid line).